
Contributions
Abstract: PB1792
Type: Publication Only
Background
Primary central nervous system lymphoma (PCNSL) is an uncommon variant of non-Hodgkin lymphoma, being immunodeficiency its main predisposing factor. Without treatment, the evolution of PCNSL is rapidly fatal. Published data support the use of penetrating chemotherapy in CNS to avoid the side effects of radiotherapy. In this sense, the GELTAMO group developed in February 2015 a protocol with carmustine, rituximab, cytarabine and high dose methotrexate (BRAM) followed by high-dose chemotherapy with carmustine and thiotepa and autologous stem cell transplant rescue (ASCT).
Aims
This retrospective study aimed to analyse the characteristics and outcomes of patients diagnosed of PCNSL in our institution since the implementation of the 2015 GELTAMO protocol.
Methods
All adult (>16 years) patients with PCNSL diagnosed in our centre from February 2015 to February 2018 were identified. Clinical, histopathological and cytogenetic data at diagnosis were collected, as well as information of treatment outcomes and toxicities.
Results
Between February 2015 and February 2017, 7 patients were diagnosed of PCNSL. Their clinic and histologic characteristics are shown in table 1.
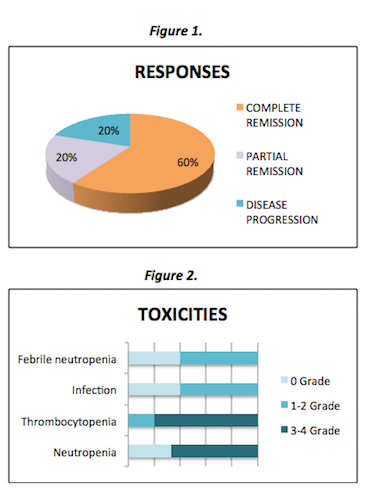
Two patients were managed by palliative care because of their age (above 74 years) and general condition (ECOG >3); they died within 6 months from diagnosis.The remaining 5 patients received 2 cycles of BRAM according to the 2015 GELTAMO protocol.Three patients (60%) received BRAM full doses and all of them achieved complete remission.Two patients (40%) required dose reduction of methotrexate and cytarabine due to treatment related toxicities and advanced age respectively. Of these, one reached stable disease and required radiotherapy prior to ASCT and one reached complete remission and did not receive ASCT but relapsed after 4 months.Two patients (40%) did not undergo ASCT because of comorbidities, being now one in relapse and one in complete remission.Of the three patients who received ASCT (60%), two achieved complete remission and one is in now in the ASCT procedure.The responses and mayor toxicities are shown in figures 1 and 2 respectively.Toxicities were mainly hematologic, infectious and renal. They were manageable, but forced to reduce doses in one patient.Their progression free survivals are in the range of 9 to 20 months.
Table 1. Patient Characteristics (n= 7)
Gender-no. (%) Female Male |
2(29) 5(71) |
| Median age- yr (range) | 66 (52-76) |
Diagnosis-no. (%) - DLBCL - High grade B cell lymphoma, NOS |
5(71) 2(29) |
| ECOG median (range) | 1(1-4) |
| Immunodeficiency-no. (%) | 0 (0) |
Intracranial lesion-no. (%) - solitary - multiple - periventricular - non periventricular |
4(57) 3(43) 3(43) 4(57) |
Immunohistochemistry- no. - Bcl6 - Bcl2 - MUM1 - c-myc - ki-67>80% |
7 7 6 2 7 |
Treatment- no. (%) Palliative BRAMx2 cycles BRAMx2 cycles + autologous HCT Radiotherapy prior to autologous HCT |
2 (29) 2 (29) 3(43) 1 (14) |
Conclusion
PCNSL continues to be a challenge for haematologists. Our results show that the 2015 GELTAMO protocol is an effective and feasible treatment scheme. Toxicities were manageable, being grade 2-3 haematological toxicity the most frequent complication.The ASCT role in the duration of remission and survival should be evaluated with longer follow-up.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): CNS lymphoma
Abstract: PB1792
Type: Publication Only
Background
Primary central nervous system lymphoma (PCNSL) is an uncommon variant of non-Hodgkin lymphoma, being immunodeficiency its main predisposing factor. Without treatment, the evolution of PCNSL is rapidly fatal. Published data support the use of penetrating chemotherapy in CNS to avoid the side effects of radiotherapy. In this sense, the GELTAMO group developed in February 2015 a protocol with carmustine, rituximab, cytarabine and high dose methotrexate (BRAM) followed by high-dose chemotherapy with carmustine and thiotepa and autologous stem cell transplant rescue (ASCT).
Aims
This retrospective study aimed to analyse the characteristics and outcomes of patients diagnosed of PCNSL in our institution since the implementation of the 2015 GELTAMO protocol.
Methods
All adult (>16 years) patients with PCNSL diagnosed in our centre from February 2015 to February 2018 were identified. Clinical, histopathological and cytogenetic data at diagnosis were collected, as well as information of treatment outcomes and toxicities.
Results
Between February 2015 and February 2017, 7 patients were diagnosed of PCNSL. Their clinic and histologic characteristics are shown in table 1.
Two patients were managed by palliative care because of their age (above 74 years) and general condition (ECOG >3); they died within 6 months from diagnosis.The remaining 5 patients received 2 cycles of BRAM according to the 2015 GELTAMO protocol.Three patients (60%) received BRAM full doses and all of them achieved complete remission.Two patients (40%) required dose reduction of methotrexate and cytarabine due to treatment related toxicities and advanced age respectively. Of these, one reached stable disease and required radiotherapy prior to ASCT and one reached complete remission and did not receive ASCT but relapsed after 4 months.Two patients (40%) did not undergo ASCT because of comorbidities, being now one in relapse and one in complete remission.Of the three patients who received ASCT (60%), two achieved complete remission and one is in now in the ASCT procedure.The responses and mayor toxicities are shown in figures 1 and 2 respectively.Toxicities were mainly hematologic, infectious and renal. They were manageable, but forced to reduce doses in one patient.Their progression free survivals are in the range of 9 to 20 months.
Table 1. Patient Characteristics (n= 7)
Gender-no. (%) Female Male |
2(29) 5(71) |
| Median age- yr (range) | 66 (52-76) |
Diagnosis-no. (%) - DLBCL - High grade B cell lymphoma, NOS |
5(71) 2(29) |
| ECOG median (range) | 1(1-4) |
| Immunodeficiency-no. (%) | 0 (0) |
Intracranial lesion-no. (%) - solitary - multiple - periventricular - non periventricular |
4(57) 3(43) 3(43) 4(57) |
Immunohistochemistry- no. - Bcl6 - Bcl2 - MUM1 - c-myc - ki-67>80% |
7 7 6 2 7 |
Treatment- no. (%) Palliative BRAMx2 cycles BRAMx2 cycles + autologous HCT Radiotherapy prior to autologous HCT |
2 (29) 2 (29) 3(43) 1 (14) |

Conclusion
PCNSL continues to be a challenge for haematologists. Our results show that the 2015 GELTAMO protocol is an effective and feasible treatment scheme. Toxicities were manageable, being grade 2-3 haematological toxicity the most frequent complication.The ASCT role in the duration of remission and survival should be evaluated with longer follow-up.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): CNS lymphoma


