
Contributions
Abstract: PB2329
Type: Publication Only
Background
Blocking the PD-1 immune checkpoint is a therapeutic strategy with unprecedented clinical efficacy in the treatment of advanced cancers including hematological malignancies. Evaluation of PD1 expression in lymphoma biopsies has been studied using immunohistochemistry.
Aims
The aim of the present study was to assess the expression of PD-1 in lymph node suspensions of 67 patients with lymphomas compared to reactive and metastatic lymph nodes using flow cytometry (FCM).
Methods
Lymph node biopsies were obtained at diagnosis from 16 patients with diffuse large B-cell lymphoma (DLBCL), 11 patients with follicular lymphoma (FL), 27 patients with Hodgkin lymphoma (HL), 6 patients with metastatic carcinomas and 7 patients with reactive lymph nodes. Lymph node suspensions were prepared by mechanical disaggregation of solid tissue using the Medimachine system (BD Biosciences). PD-1 (CD279, clone EH1 2.1, BD Biosciences) expression was assessed on CD3+, CD4+ and CD8+ T-lymphocytes. Fluorescence Minus One (FMO) was used in setting the gate for positive events. Data were acquired on BD FACSCantoII flow cytometer (BD Biosciences) and analyzed using BD FACSDiva software (BD Biosciences). Data were expressed as percentage of expressing cells and median fluorescence intensity (MFI).
Results
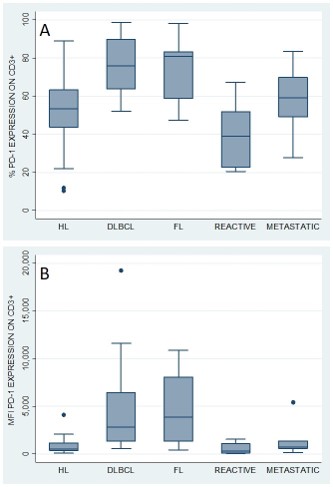
PD-1 was expressed on 53% (median, range: 10% to 89%) of tumor-infiltrating T cells in HL, which was not statistically different from PD1 expression on T cells from reactive (39%, p=0.149) and metastatic lymph nodes (59%, p=0.241). In contrast, T cells from lymph node biopsies from both DLBCL (n=16) and FL (n=11) showed higher expression of PD-1 on CD3+ T-cells (76% and 81%, respectively), when compared to both HL (53%, p<0.01) and to reactive lymph nodes (39%, p<0.01) (Fig. A). The higher PD-1 expression on CD3+ population of DLBCL and FL was also confirmed when analyzing the median fluorescence intensity. MFI on tumor-infiltrating T cells was 2855 (median, range: 582 to 19238) in DLBCL and 3889 (median, range: 451 to 10895) in FL, while it was only 536 (median, range: 88 to 4085) in HL and 339 (median, range: 69 to 1573) in reactive lymph nodes (p<0.001) (Fig. B). The pattern of PD1 expression on the CD3+ T cells was stable across CD4+ and CD8+ T-cells subpopulations in all samples. No significant difference was observed in PD1 expression on tumor-infiltrating T cells between DLBCL and FL.
Conclusion
Flow cytometric evaluation yields rapid and quantifiable information on PD1 expression of tumor-infiltrating T cells. We found strong PD1 expression on tumor-infiltrating T cells in lymph node biopsies of patients with newly diagnosed B-cell Non-Hodgkin lymphomas, both of diffuse large B cell and follicular type. This expression was stronger than on T cells from HL biopsies and reactive lymph nodes. Further studies are needed to assess the utility of flow cytometric evaluation of PD1 expression as a biomarker for immune checkpoint inhibition.
Session topic: 19. Non-Hodgkin lymphoma Biology & Translational Research
Keyword(s): DLBCL, flow cytometry, Follicular lymphoma
Abstract: PB2329
Type: Publication Only
Background
Blocking the PD-1 immune checkpoint is a therapeutic strategy with unprecedented clinical efficacy in the treatment of advanced cancers including hematological malignancies. Evaluation of PD1 expression in lymphoma biopsies has been studied using immunohistochemistry.
Aims
The aim of the present study was to assess the expression of PD-1 in lymph node suspensions of 67 patients with lymphomas compared to reactive and metastatic lymph nodes using flow cytometry (FCM).
Methods
Lymph node biopsies were obtained at diagnosis from 16 patients with diffuse large B-cell lymphoma (DLBCL), 11 patients with follicular lymphoma (FL), 27 patients with Hodgkin lymphoma (HL), 6 patients with metastatic carcinomas and 7 patients with reactive lymph nodes. Lymph node suspensions were prepared by mechanical disaggregation of solid tissue using the Medimachine system (BD Biosciences). PD-1 (CD279, clone EH1 2.1, BD Biosciences) expression was assessed on CD3+, CD4+ and CD8+ T-lymphocytes. Fluorescence Minus One (FMO) was used in setting the gate for positive events. Data were acquired on BD FACSCantoII flow cytometer (BD Biosciences) and analyzed using BD FACSDiva software (BD Biosciences). Data were expressed as percentage of expressing cells and median fluorescence intensity (MFI).
Results
PD-1 was expressed on 53% (median, range: 10% to 89%) of tumor-infiltrating T cells in HL, which was not statistically different from PD1 expression on T cells from reactive (39%, p=0.149) and metastatic lymph nodes (59%, p=0.241). In contrast, T cells from lymph node biopsies from both DLBCL (n=16) and FL (n=11) showed higher expression of PD-1 on CD3+ T-cells (76% and 81%, respectively), when compared to both HL (53%, p<0.01) and to reactive lymph nodes (39%, p<0.01) (Fig. A). The higher PD-1 expression on CD3+ population of DLBCL and FL was also confirmed when analyzing the median fluorescence intensity. MFI on tumor-infiltrating T cells was 2855 (median, range: 582 to 19238) in DLBCL and 3889 (median, range: 451 to 10895) in FL, while it was only 536 (median, range: 88 to 4085) in HL and 339 (median, range: 69 to 1573) in reactive lymph nodes (p<0.001) (Fig. B). The pattern of PD1 expression on the CD3+ T cells was stable across CD4+ and CD8+ T-cells subpopulations in all samples. No significant difference was observed in PD1 expression on tumor-infiltrating T cells between DLBCL and FL.

Conclusion
Flow cytometric evaluation yields rapid and quantifiable information on PD1 expression of tumor-infiltrating T cells. We found strong PD1 expression on tumor-infiltrating T cells in lymph node biopsies of patients with newly diagnosed B-cell Non-Hodgkin lymphomas, both of diffuse large B cell and follicular type. This expression was stronger than on T cells from HL biopsies and reactive lymph nodes. Further studies are needed to assess the utility of flow cytometric evaluation of PD1 expression as a biomarker for immune checkpoint inhibition.
Session topic: 19. Non-Hodgkin lymphoma Biology & Translational Research
Keyword(s): DLBCL, flow cytometry, Follicular lymphoma


