
Contributions
Abstract: PB2002
Type: Publication Only
Background
The management of patients with refractory or relapsed Hodgkin lymphoma (HL) remains controversial. A standard salvage therapy is DHAP chemotherapy treatment (dexamethasone, high-dose cytarabine, cisplatin) followed by autologous stem cells transplant (ASCT) which obtains an ORR of about 50%. Bendamustine has demonstrated efficacy in several lymphoproliferative disorders but limited data are available regarding the schedule in patients with HL, in particular its dosage and possible combinations. Brentuximab Vedotin is a CD30-directed antibody-drug conjugate, currently approved for the treatment of relapsed or refractory HL.
Aims
The objective of this prospective study is to evaluate the efficacy and safety of the salvage cytotoxic regimen with Bendamustine and Brentuximab association in patients with refractory and/or relapsed HL.
Methods
Patients with relapsed or refractory classical HL from September 2013 to November 2017 were enrolled in the prospective study receiving Brentuximab 1.8 mg/kg every 21 days at day 1 associated with Bendamustine 120 mg/mq every 21 days at day 1 and 2 (Bv+B schedule). Dose reductions and/or treatment delay were recorded. The treatment efficacy was evaluated according to the Revised Response Criteria for Malignant Lymphoma. The Overall Response Rate (ORR), Overall Survival (OS) and EFS (Event Free Survival) were analyzed. Any adverse event occurred was recorded and classified for type and grade using NCI-CTCAE criteria (v 4.0).
Results
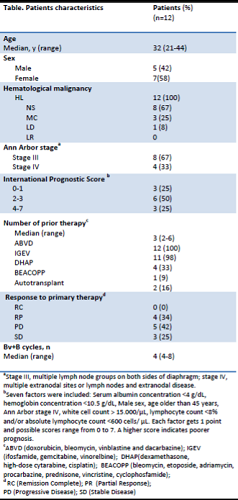
Twelve consecutive patients (5 M/7 F) with a median age of 32 years (21-44) received this salvage regimen treatment (Bv+B). All patients had an advanced stage disease, with 3 patients having stage IV disease. The median number of prior therapies was 3 (range 2-6) and all patients received ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) as upfront therapy. All patients received Bv+B schedule as salvage therapy and achieved a complete response (ORR 100%). Ten patients underwent a stem cell transplant (8 autologous and 2 haploidentical), while two patients are currently in follow-up.
All patients received primary prophylaxis for chemotherapy related neutropenia with G-CSF.
Three patients (25%) had a grade 1 infusion reaction with fever and a skin rash, managed with corticosteroid injections and a successful antihistamine plus corticosteroid prophylaxis in the subsequent cycles of treatment; 4 patients (33%) had a citomegalovirus reactivation treated with Valganciclovir (450 mg twice a day); 2 patients had a grade 1 peripheral neuropathy. A dose reduction of 20% for bendamustine and 10 % of brentuximab vedotin was required for 4 patients (33%). No patient had to delay chemotherapy treatment and all patients respected the treatment time schedule. EFS was 90% at 24 months, for a median follow up of 12 months, as one patient did not maintain the complete response and received subsequent salvage treatment. OS was 100%.
Conclusion
High-dose bendamustine plus brentuximab have shown relevant efficacy and a relatively good safety profile in a setting of heavily pretreated patients with HL. Adequate monitoring of CMV reactivation is recommended. This combination could be considered as a bridge to first or second autologous or allogenic SCT. However, these results should be validated by controlled and prospective studies involving larger number of patients.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): Autologous bone marrow transplant, bendamustine, Hodgkin's disease, Refractory
Abstract: PB2002
Type: Publication Only
Background
The management of patients with refractory or relapsed Hodgkin lymphoma (HL) remains controversial. A standard salvage therapy is DHAP chemotherapy treatment (dexamethasone, high-dose cytarabine, cisplatin) followed by autologous stem cells transplant (ASCT) which obtains an ORR of about 50%. Bendamustine has demonstrated efficacy in several lymphoproliferative disorders but limited data are available regarding the schedule in patients with HL, in particular its dosage and possible combinations. Brentuximab Vedotin is a CD30-directed antibody-drug conjugate, currently approved for the treatment of relapsed or refractory HL.
Aims
The objective of this prospective study is to evaluate the efficacy and safety of the salvage cytotoxic regimen with Bendamustine and Brentuximab association in patients with refractory and/or relapsed HL.
Methods
Patients with relapsed or refractory classical HL from September 2013 to November 2017 were enrolled in the prospective study receiving Brentuximab 1.8 mg/kg every 21 days at day 1 associated with Bendamustine 120 mg/mq every 21 days at day 1 and 2 (Bv+B schedule). Dose reductions and/or treatment delay were recorded. The treatment efficacy was evaluated according to the Revised Response Criteria for Malignant Lymphoma. The Overall Response Rate (ORR), Overall Survival (OS) and EFS (Event Free Survival) were analyzed. Any adverse event occurred was recorded and classified for type and grade using NCI-CTCAE criteria (v 4.0).
Results
Twelve consecutive patients (5 M/7 F) with a median age of 32 years (21-44) received this salvage regimen treatment (Bv+B). All patients had an advanced stage disease, with 3 patients having stage IV disease. The median number of prior therapies was 3 (range 2-6) and all patients received ABVD (doxorubicin, bleomycin, vinblastine, dacarbazine) as upfront therapy. All patients received Bv+B schedule as salvage therapy and achieved a complete response (ORR 100%). Ten patients underwent a stem cell transplant (8 autologous and 2 haploidentical), while two patients are currently in follow-up.
All patients received primary prophylaxis for chemotherapy related neutropenia with G-CSF.
Three patients (25%) had a grade 1 infusion reaction with fever and a skin rash, managed with corticosteroid injections and a successful antihistamine plus corticosteroid prophylaxis in the subsequent cycles of treatment; 4 patients (33%) had a citomegalovirus reactivation treated with Valganciclovir (450 mg twice a day); 2 patients had a grade 1 peripheral neuropathy. A dose reduction of 20% for bendamustine and 10 % of brentuximab vedotin was required for 4 patients (33%). No patient had to delay chemotherapy treatment and all patients respected the treatment time schedule. EFS was 90% at 24 months, for a median follow up of 12 months, as one patient did not maintain the complete response and received subsequent salvage treatment. OS was 100%.

Conclusion
High-dose bendamustine plus brentuximab have shown relevant efficacy and a relatively good safety profile in a setting of heavily pretreated patients with HL. Adequate monitoring of CMV reactivation is recommended. This combination could be considered as a bridge to first or second autologous or allogenic SCT. However, these results should be validated by controlled and prospective studies involving larger number of patients.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): Autologous bone marrow transplant, bendamustine, Hodgkin's disease, Refractory


