
Contributions
Abstract: PB1999
Type: Publication Only
Background
Classical Hodgkin lymphoma (cHL) is a hematologic malignancy with poor prognosis for advanced-stage patients who do not respond well to frontline (FL) therapy.
Aims
This study examines patient characteristics and clinical outcomes associated with FL systemic regimens used to treat advanced-stage cHL in the United Kingdom (UK), France (FRA), and Germany (DE).
Methods
Hematologists and oncologists (N=57) from the UK, FRA, and DE retrospectively identified patients diagnosed with advanced stage cHL and treated with FL systemic therapy: doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD); doxorubicin, vinblastine, and dacarbazine (AVD); dose-escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPescalated); BEACOPP. Descriptive statistics examined patient characteristics, FL regimens, and associated adverse events (AEs). Bivariate analyses (ANOVA, chi-square, or Fisher’s exact test) compared patient characteristics by regimen. Univariate analyses and bivariate analyses (chi-square or Fisher’s exact test) compared clinical outcomes by regimen. Progression-free survival (PFS) was assessed with Kaplan Meier curves.
Results
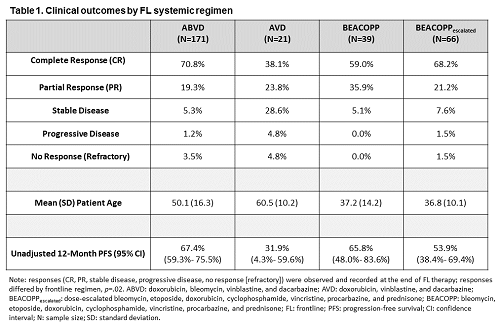
Mean (SD) age at initial cHL diagnosis was 46.2 (16.2) for the aggregate sample (N=297), which was mostly male (65.0%), evenly distributed across the UK (32.3%), FRA (34.3%), and DE (33.3%), comprised of patients initially diagnosed with advanced-stage cHL (Stage IIb 19.2%, Stage III 35.1%, Stage IV 45.7%), and patients treated in FL with ABVD (57.6%), AVD (7.1%), BEACOPP (13.1%), or BEACOPPescalated (22.2%). Among patients who received ABVD, AVD, BEACOPP, and BEACOPPescalated, administration of FL systemic regimen type differed by patient mean [SD] age at initial cHL diagnosis (50.1 [16.3], 60.5 [10.2], 37.2 [14.2], 36.8 [10.1], respectively), p<.001, and by country (UK 45.0%, FRA 35.1%, DE 19.9%), (UK 52.4%, FRA 9.5%, DE 38.1%), (UK 10.3%, FRA 43.6%, DE 46.2%), (UK 6.1%, FRA 34.9%, DE 59.1%), respectively, p<.001. Among patients who received ABVD, AVD, BEACOPP, and BEACOPPescalated, AEs included, but were not limited to alopecia (46.2%, 38.1%, 48.7%, 81.8%, respectively), neutropenia (22.2%, 4.8%, 28.2%, 51.5%, respectively), infection (7.0%, 9.5%, 5.1%, 18.2%, respectively), and peripheral neuropathy (4.1%, 4.8%, 7.7%, 1.5%, respectively). Response at end of FL therapy differed within the aggregate sample (complete response [CR] 66.3%; partial response [PR] 22.2%; stable disease 7.4%; progressive disease 1.4%; “no response” [refractory] 2.7%), p<.001. Responses by FL regimen are presented in Table 1. Median follow-up was 8.8 months (range: 0-64 months). Unadjusted 12-month PFS by FL regimen is presented in Table 1.
Conclusion
ABVD and AVD were more commonly administered for older patients, whereas BEACOPP and BEACOPPescalated were more commonly administered for younger patients. Alopecia, neutropenia, and infection were most commonly observed in BEACOPPescalated patients, whereas peripheral neuropathy was most commonly observed in BEACOPP patients. CR at end of FL therapy was more common in ABVD and BEACOPPescalated patients than in AVD and BEACOPP patients in this retrospective analysis. These findings demonstrate that treatment outcomes in the real-world practice setting may be different than those observed in clinical trials. These findings underscore the unmet need in patients with cHL and the importance of novel treatments.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): chemotherapy, Clinical outcome, Hodgkin's Lymphoma
Abstract: PB1999
Type: Publication Only
Background
Classical Hodgkin lymphoma (cHL) is a hematologic malignancy with poor prognosis for advanced-stage patients who do not respond well to frontline (FL) therapy.
Aims
This study examines patient characteristics and clinical outcomes associated with FL systemic regimens used to treat advanced-stage cHL in the United Kingdom (UK), France (FRA), and Germany (DE).
Methods
Hematologists and oncologists (N=57) from the UK, FRA, and DE retrospectively identified patients diagnosed with advanced stage cHL and treated with FL systemic therapy: doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD); doxorubicin, vinblastine, and dacarbazine (AVD); dose-escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (BEACOPPescalated); BEACOPP. Descriptive statistics examined patient characteristics, FL regimens, and associated adverse events (AEs). Bivariate analyses (ANOVA, chi-square, or Fisher’s exact test) compared patient characteristics by regimen. Univariate analyses and bivariate analyses (chi-square or Fisher’s exact test) compared clinical outcomes by regimen. Progression-free survival (PFS) was assessed with Kaplan Meier curves.
Results
Mean (SD) age at initial cHL diagnosis was 46.2 (16.2) for the aggregate sample (N=297), which was mostly male (65.0%), evenly distributed across the UK (32.3%), FRA (34.3%), and DE (33.3%), comprised of patients initially diagnosed with advanced-stage cHL (Stage IIb 19.2%, Stage III 35.1%, Stage IV 45.7%), and patients treated in FL with ABVD (57.6%), AVD (7.1%), BEACOPP (13.1%), or BEACOPPescalated (22.2%). Among patients who received ABVD, AVD, BEACOPP, and BEACOPPescalated, administration of FL systemic regimen type differed by patient mean [SD] age at initial cHL diagnosis (50.1 [16.3], 60.5 [10.2], 37.2 [14.2], 36.8 [10.1], respectively), p<.001, and by country (UK 45.0%, FRA 35.1%, DE 19.9%), (UK 52.4%, FRA 9.5%, DE 38.1%), (UK 10.3%, FRA 43.6%, DE 46.2%), (UK 6.1%, FRA 34.9%, DE 59.1%), respectively, p<.001. Among patients who received ABVD, AVD, BEACOPP, and BEACOPPescalated, AEs included, but were not limited to alopecia (46.2%, 38.1%, 48.7%, 81.8%, respectively), neutropenia (22.2%, 4.8%, 28.2%, 51.5%, respectively), infection (7.0%, 9.5%, 5.1%, 18.2%, respectively), and peripheral neuropathy (4.1%, 4.8%, 7.7%, 1.5%, respectively). Response at end of FL therapy differed within the aggregate sample (complete response [CR] 66.3%; partial response [PR] 22.2%; stable disease 7.4%; progressive disease 1.4%; “no response” [refractory] 2.7%), p<.001. Responses by FL regimen are presented in Table 1. Median follow-up was 8.8 months (range: 0-64 months). Unadjusted 12-month PFS by FL regimen is presented in Table 1.

Conclusion
ABVD and AVD were more commonly administered for older patients, whereas BEACOPP and BEACOPPescalated were more commonly administered for younger patients. Alopecia, neutropenia, and infection were most commonly observed in BEACOPPescalated patients, whereas peripheral neuropathy was most commonly observed in BEACOPP patients. CR at end of FL therapy was more common in ABVD and BEACOPPescalated patients than in AVD and BEACOPP patients in this retrospective analysis. These findings demonstrate that treatment outcomes in the real-world practice setting may be different than those observed in clinical trials. These findings underscore the unmet need in patients with cHL and the importance of novel treatments.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): chemotherapy, Clinical outcome, Hodgkin's Lymphoma


