
Contributions
Abstract: PB2185
Type: Publication Only
Background
Patients with transplant-ineligible, newly diagnosed multiple myeloma (NDMM) require therapies which prolong survival and improve quality of life. The combination of bortezomib (V), lenalidomide (R), and dexamethasone (d) significantly improves progression-free (PFS) and overall survival compared with Rd in NDMM, and has an acceptable safety profile. Combining VRd with a monoclonal antibody (mAb) may further improve efficacy. Isatuximab (ISA) is an anti-CD38 mAb that demonstrates antitumor and immunomodulatory activities with strong potentiation when combined with V and R in MM xenograft models.
Aims
This Phase III, randomized, open-label, multicenter study (NCT03319667; IMROZ), is being conducted to evaluate the clinical benefit of ISA plus VRd versus VRd alone in the treatment of adult patients with transplant-ineligible NDMM.
Methods
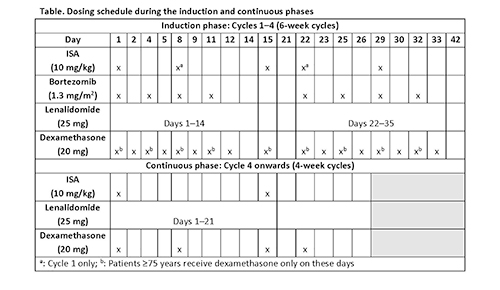
All enrolled patients will provide written informed consent. Approximately 440 patients with symptomatic MM (International Myeloma Working Group [IMWG] criteria) will be randomly assigned, according to Revised International Staging System criteria (I or II vs III vs unknown) and age (<70 vs ≥70 years), in a 3:2 ratio to ISA 10 mg/kg plus VRd or VRd alone for four 6-week cycles (induction phase) (Table). After Cycle 4, patients will receive Rd with or without ISA in 4-week cycles until disease progression, unacceptable adverse events (AEs), or patient decision to discontinue (Table) (continuous phase). ISA will be reduced to monthly dosing from Cycle 18. Patients in VRd arm who progress during the continuous phase could be eligible for cross-over to ISA plus Rd. The primary endpoint is PFS, which is defined as the time from randomization to the date of disease progression (assessed by a blinded independent review committee according to IMWG criteria) or death, and will be compared between the two arms with a 1-sided stratified log-rank test. Key secondary endpoints will include rate of very good partial response or better, minimal residual disease negativity rate, and complete response rate. Safety evaluations include AEs, laboratory parameters, vital signs, and physical examination.
Results
The IMROZ study is currently enrolling patients; recruitment is planned in approximately 100 sites worldwide, including Japan and China.
Conclusion
This Phase III, randomized, multicenter study will provide an evaluation of the efficacy and safety of ISA plus VRd in patients with transplant-ineligible NDMM.
Funding: Sanofi
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): CD38, Monoclonal antibody, Multiple Myeloma
Abstract: PB2185
Type: Publication Only
Background
Patients with transplant-ineligible, newly diagnosed multiple myeloma (NDMM) require therapies which prolong survival and improve quality of life. The combination of bortezomib (V), lenalidomide (R), and dexamethasone (d) significantly improves progression-free (PFS) and overall survival compared with Rd in NDMM, and has an acceptable safety profile. Combining VRd with a monoclonal antibody (mAb) may further improve efficacy. Isatuximab (ISA) is an anti-CD38 mAb that demonstrates antitumor and immunomodulatory activities with strong potentiation when combined with V and R in MM xenograft models.
Aims
This Phase III, randomized, open-label, multicenter study (NCT03319667; IMROZ), is being conducted to evaluate the clinical benefit of ISA plus VRd versus VRd alone in the treatment of adult patients with transplant-ineligible NDMM.
Methods
All enrolled patients will provide written informed consent. Approximately 440 patients with symptomatic MM (International Myeloma Working Group [IMWG] criteria) will be randomly assigned, according to Revised International Staging System criteria (I or II vs III vs unknown) and age (<70 vs ≥70 years), in a 3:2 ratio to ISA 10 mg/kg plus VRd or VRd alone for four 6-week cycles (induction phase) (Table). After Cycle 4, patients will receive Rd with or without ISA in 4-week cycles until disease progression, unacceptable adverse events (AEs), or patient decision to discontinue (Table) (continuous phase). ISA will be reduced to monthly dosing from Cycle 18. Patients in VRd arm who progress during the continuous phase could be eligible for cross-over to ISA plus Rd. The primary endpoint is PFS, which is defined as the time from randomization to the date of disease progression (assessed by a blinded independent review committee according to IMWG criteria) or death, and will be compared between the two arms with a 1-sided stratified log-rank test. Key secondary endpoints will include rate of very good partial response or better, minimal residual disease negativity rate, and complete response rate. Safety evaluations include AEs, laboratory parameters, vital signs, and physical examination.
Results
The IMROZ study is currently enrolling patients; recruitment is planned in approximately 100 sites worldwide, including Japan and China.

Conclusion
This Phase III, randomized, multicenter study will provide an evaluation of the efficacy and safety of ISA plus VRd in patients with transplant-ineligible NDMM.
Funding: Sanofi
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): CD38, Monoclonal antibody, Multiple Myeloma


