
Contributions
Abstract: PB2176
Type: Publication Only
Background
Approval of novel regimens for multiple myeloma (MM) in Europe have improved the efficacy and safety of treating patients with MM. Peripheral neuropathy (PN) is both a MM complication and a toxicity that can occur from some anti-myeloma treatments. Treatment-induced peripheral neuropathy (TIPN) impairs quality of life of patients; however, the economic burden of TIPN is not well known.
Aims
The primary aim of the study was to understand the treatment patterns in patients with MM. This analysis focused on secondary and exploratory objectives assessing the occurrence and economic burden of TIPN in a real-world setting in Sweden.
Methods
This retrospective cohort study used data from electronic medical records (EMR) from three large haematology clinics in Stockholm, Sweden that were linked to national health registries. Eligible patients were those with a diagnosis of MM (ICD-10:CD90.0) in the Swedish Cancer Registry between 2006–2015 and had initiated MM treatment during the same period. Follow-up was until last EMR visit, death or study end (March 2017). For this analysis, patients who had no record of PN or TIPN for the past two years before the initiation of the analysed treatment line were included. Diagnosis of TIPN was retrieved from the National Patient Register (ICD-10:G62.0) and EMR case notes. An index matching method with replacement was used to match patients with TIPN and those without for baseline characteristics, MM treatment that induced TIPN and line of therapy. For patients with TIPN, the time from treatment initiation to the TIPN diagnosis date was estimated (TIPN lag days). For patients without TIPN, a pseudo TIPN diagnosis date was set according to the TIPN lag days of the related case, after the initiation of the matched treatment. Cost calculations were based on 2015 DRG tariffs. Statistical analyses were descriptive in nature.
Results
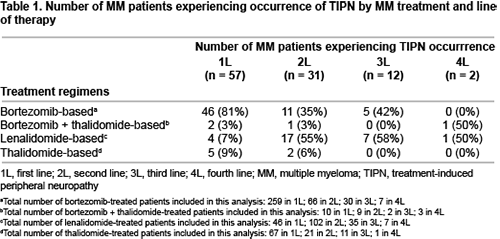
In total, 1298 MM patients were identified from EMRs; of which 550 were eligible for the overall study. Of these patients, 385 met this analysis criterion (135 received stem cell transplantation [SCT] and 250 did not [non-SCT]). Overall, 26% of patients were diagnosed with TIPN. At first line (1L), most patients with TIPN had received a bortezomib-based regimen (81%) while at second line (2L), most patients with TIPN had received a lenalidomide-based regimen (55%) (Table 1). Median TIPN lag days was similar in both SCT and non-SCT patients (4.3 and 4.4 months respectively, for 1L bortezomib-based treatment and 1.3 months each, for 2L lenalidomide-based treatment). Matched patients (n=73) were well balanced on all matching variables. Median follow-up from TIPN date and pseudo date was 1.7 and 1.8 years, respectively. Overall, patients with TIPN had increased healthcare resource utilisation (HRU) compared with those without TIPN: mean hospital inpatient visits (3.5 vs 2.3 visits), median total length of stay (7.2 vs 1.2 days) and mean hospital outpatient visits (17.8 vs 12.6 visits). Similarly, patients with TIPN showed significant increased total cost of HRU per patient-year than those without TIPN (mean €52,353 vs €31,194, respectively), mainly driven by outpatient prescription drugs costs (mean €27,399 vs €18,808).
Conclusion
Findings showed that most patients with TIPN had received bortezomib-based treatments. Patients with TIPN had increased HRU and ~70% higher cost per patient-year versus those without TIPN. There is a need for effective MM treatments which reduce the occurrence of TIPN in order to ensure benefit to patients and decrease healthcare expenditure.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Adverse reaction, Cost analysis, Health care, Multiple Myeloma
Abstract: PB2176
Type: Publication Only
Background
Approval of novel regimens for multiple myeloma (MM) in Europe have improved the efficacy and safety of treating patients with MM. Peripheral neuropathy (PN) is both a MM complication and a toxicity that can occur from some anti-myeloma treatments. Treatment-induced peripheral neuropathy (TIPN) impairs quality of life of patients; however, the economic burden of TIPN is not well known.
Aims
The primary aim of the study was to understand the treatment patterns in patients with MM. This analysis focused on secondary and exploratory objectives assessing the occurrence and economic burden of TIPN in a real-world setting in Sweden.
Methods
This retrospective cohort study used data from electronic medical records (EMR) from three large haematology clinics in Stockholm, Sweden that were linked to national health registries. Eligible patients were those with a diagnosis of MM (ICD-10:CD90.0) in the Swedish Cancer Registry between 2006–2015 and had initiated MM treatment during the same period. Follow-up was until last EMR visit, death or study end (March 2017). For this analysis, patients who had no record of PN or TIPN for the past two years before the initiation of the analysed treatment line were included. Diagnosis of TIPN was retrieved from the National Patient Register (ICD-10:G62.0) and EMR case notes. An index matching method with replacement was used to match patients with TIPN and those without for baseline characteristics, MM treatment that induced TIPN and line of therapy. For patients with TIPN, the time from treatment initiation to the TIPN diagnosis date was estimated (TIPN lag days). For patients without TIPN, a pseudo TIPN diagnosis date was set according to the TIPN lag days of the related case, after the initiation of the matched treatment. Cost calculations were based on 2015 DRG tariffs. Statistical analyses were descriptive in nature.
Results
In total, 1298 MM patients were identified from EMRs; of which 550 were eligible for the overall study. Of these patients, 385 met this analysis criterion (135 received stem cell transplantation [SCT] and 250 did not [non-SCT]). Overall, 26% of patients were diagnosed with TIPN. At first line (1L), most patients with TIPN had received a bortezomib-based regimen (81%) while at second line (2L), most patients with TIPN had received a lenalidomide-based regimen (55%) (Table 1). Median TIPN lag days was similar in both SCT and non-SCT patients (4.3 and 4.4 months respectively, for 1L bortezomib-based treatment and 1.3 months each, for 2L lenalidomide-based treatment). Matched patients (n=73) were well balanced on all matching variables. Median follow-up from TIPN date and pseudo date was 1.7 and 1.8 years, respectively. Overall, patients with TIPN had increased healthcare resource utilisation (HRU) compared with those without TIPN: mean hospital inpatient visits (3.5 vs 2.3 visits), median total length of stay (7.2 vs 1.2 days) and mean hospital outpatient visits (17.8 vs 12.6 visits). Similarly, patients with TIPN showed significant increased total cost of HRU per patient-year than those without TIPN (mean €52,353 vs €31,194, respectively), mainly driven by outpatient prescription drugs costs (mean €27,399 vs €18,808).

Conclusion
Findings showed that most patients with TIPN had received bortezomib-based treatments. Patients with TIPN had increased HRU and ~70% higher cost per patient-year versus those without TIPN. There is a need for effective MM treatments which reduce the occurrence of TIPN in order to ensure benefit to patients and decrease healthcare expenditure.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Adverse reaction, Cost analysis, Health care, Multiple Myeloma


