
Contributions
Abstract: PB2088
Type: Publication Only
Background
MDS are a rare group of blood cancers that occur as a result of disordered development of blood cells within bone marrow. MDS occur most commonly in older adults, with a median age at dx of ≥65 yrs. Previous studies have shown oral RIGO at a dose of 560mg BID in Low-Risk MDS (LR-MDS) patients (pts) demonstrate a transfusion independence rate (IWG2006) of 44% (Raza et al, Blood 2017). Oral RIGO in combo with AZA in pts with MDS is being studied (Navada S, Blood 2016). In monotherapy & combination trials, oral RIGO has been associated with urinary adverse events (UAEs) of interest, shown to be dose & administration scheme dependent (Garcia-Manero G, Blood 2016).
Aims
Reported are initial results of a dose exploration study in MDS pts focusing on impact of risk-mitigation plans in reducing incidence of UAEs including hematuria.
Methods
Pts w MDS or leukemia (N=168) were given oral RIGO monotherapy in doses escalating from 70mg-1680mg 2x daily for either 14 consecutive days per 21-day cycle (intermittent schedule) or for 21 consecutive days per 21-day cycle (continuous schedule). In Part 1 of a trial of oral RIGO with AZA 75mg/m2/d SC or IV (N=54) pts were administered RIGO 2x daily on Day 1-21 of a 28-day cycle in escalating cohorts, max dose of 560mg qAM & 280mg qPM (total 840mg) & AZA, administered for 7days/month starting on Day 8. In an ongoing Part 2 Study, oral RIGO at a total dose of 1120mg in 2 cohorts 560mg BID or 840mg/280mg is administered on Days 1-21 of a 28-day cycle in MDS pts with AZA; applying risk-mitigation plans to reduce UAEs:
1. Second RIGO dose must be taken at 3 PM (±1 hour) at least 2 hours after lunch to avoid a nocturnal bladder dwell time (Maniar M, et al. ASCO 2018 Sub for Pub)
2. Oral hydration of at least two liters of fluid per day is encouraged
3. Mandatory bladder emptying prior to bedtime
4. Urine pH approx. 2 hours after AM dose. Sodium bicarbonate suggested 650 TID if pH tests < 7.5
Results
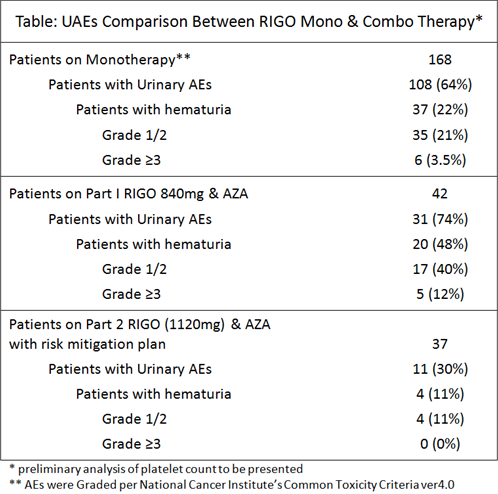
The most frequent treatment emergent AEs observed in safety-evaluable pts in MDS studies w oral RIGO monotherapy (N = 168) were urinary. Higher frequency was identified for pts treated with continuous RIGO dosing versus intermittent dosing. Due to UAEs, continuous 560mg BID dosing is no longer being studied. Incidences of renal and urinary disorders have been identified with single agent AZA. In a trial of RIGO (total dose of 840mg) & AZA, incidence of UAEs, including hematuria, was 74%, w Gr ≥3 UAEs of 29%. In 37 pts studied w oral RIGO 1120mg & AZA, implementation of risk-mitigating plans to reduce UAEs, UAEs were 30%; Gr ≥3 5% have been seen to date (Table).
Conclusion
Dose optimization & risk mitigation plans to reduce UAEs associated with oral RIGO in combination with AZA have resulted, to date, in a decrease in frequency of UAEs. This study is ongoing. Reduction of AEs permits pts to continue treatment to optimize benefit. Reduction in incidence of UAEs enables continued study of oral RIGO in LR-MDS based on promising transfusion independence rate previously reported (Raza et al, Blood 2017).
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): MDS
Abstract: PB2088
Type: Publication Only
Background
MDS are a rare group of blood cancers that occur as a result of disordered development of blood cells within bone marrow. MDS occur most commonly in older adults, with a median age at dx of ≥65 yrs. Previous studies have shown oral RIGO at a dose of 560mg BID in Low-Risk MDS (LR-MDS) patients (pts) demonstrate a transfusion independence rate (IWG2006) of 44% (Raza et al, Blood 2017). Oral RIGO in combo with AZA in pts with MDS is being studied (Navada S, Blood 2016). In monotherapy & combination trials, oral RIGO has been associated with urinary adverse events (UAEs) of interest, shown to be dose & administration scheme dependent (Garcia-Manero G, Blood 2016).
Aims
Reported are initial results of a dose exploration study in MDS pts focusing on impact of risk-mitigation plans in reducing incidence of UAEs including hematuria.
Methods
Pts w MDS or leukemia (N=168) were given oral RIGO monotherapy in doses escalating from 70mg-1680mg 2x daily for either 14 consecutive days per 21-day cycle (intermittent schedule) or for 21 consecutive days per 21-day cycle (continuous schedule). In Part 1 of a trial of oral RIGO with AZA 75mg/m2/d SC or IV (N=54) pts were administered RIGO 2x daily on Day 1-21 of a 28-day cycle in escalating cohorts, max dose of 560mg qAM & 280mg qPM (total 840mg) & AZA, administered for 7days/month starting on Day 8. In an ongoing Part 2 Study, oral RIGO at a total dose of 1120mg in 2 cohorts 560mg BID or 840mg/280mg is administered on Days 1-21 of a 28-day cycle in MDS pts with AZA; applying risk-mitigation plans to reduce UAEs:
1. Second RIGO dose must be taken at 3 PM (±1 hour) at least 2 hours after lunch to avoid a nocturnal bladder dwell time (Maniar M, et al. ASCO 2018 Sub for Pub)
2. Oral hydration of at least two liters of fluid per day is encouraged
3. Mandatory bladder emptying prior to bedtime
4. Urine pH approx. 2 hours after AM dose. Sodium bicarbonate suggested 650 TID if pH tests < 7.5
Results
The most frequent treatment emergent AEs observed in safety-evaluable pts in MDS studies w oral RIGO monotherapy (N = 168) were urinary. Higher frequency was identified for pts treated with continuous RIGO dosing versus intermittent dosing. Due to UAEs, continuous 560mg BID dosing is no longer being studied. Incidences of renal and urinary disorders have been identified with single agent AZA. In a trial of RIGO (total dose of 840mg) & AZA, incidence of UAEs, including hematuria, was 74%, w Gr ≥3 UAEs of 29%. In 37 pts studied w oral RIGO 1120mg & AZA, implementation of risk-mitigating plans to reduce UAEs, UAEs were 30%; Gr ≥3 5% have been seen to date (Table).

Conclusion
Dose optimization & risk mitigation plans to reduce UAEs associated with oral RIGO in combination with AZA have resulted, to date, in a decrease in frequency of UAEs. This study is ongoing. Reduction of AEs permits pts to continue treatment to optimize benefit. Reduction in incidence of UAEs enables continued study of oral RIGO in LR-MDS based on promising transfusion independence rate previously reported (Raza et al, Blood 2017).
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): MDS


