
Contributions
Abstract: PB2075
Type: Publication Only
Background
In myelodysplastic syndromes (MDS) deregulation of immune effectors pathogenetically drives abnormal haemopoiesis and enhanced leukemic propensity. Human leukocyte antigen G (HLA-G) is a nonclassical MHC class I antigen, regularly not expressed in normal tissues except in trophoblasts from early gestation placentas and other immune-privileged tissues. Evidence for HLA-G silencing by a DNA methylation process has been reported. Interestingly, the use of demethylating agents such as 5-azacytidine further demonstrated that the repression of HLA-G gene activity in cultured various cell lines is reversed by demethylating treatment. HLA-G primary transcript generates seven alternative mRNAs that encode membrane-bound HLA-G1, -G2, -G3, -G4 and soluble HLA-G5,-G6 and –G7 isoforms. HLA-G has a direct inhibitory effect on the cytolytic activity of NK cells, cytotoxic T lymphocytes and is implicated in the induction of Foxp3-regulatory T cells. Therefore, HLA-G possesses immune tolerogenic activity with potential implications in antitumor immune responses.
Aims
In order to investigate potential implication of HLA-G in immune deregulation underlying MDS pathogenesis and evolution we studied HLA-G gene and protein expression in CD34 cells of patients with primary MDS.
Methods
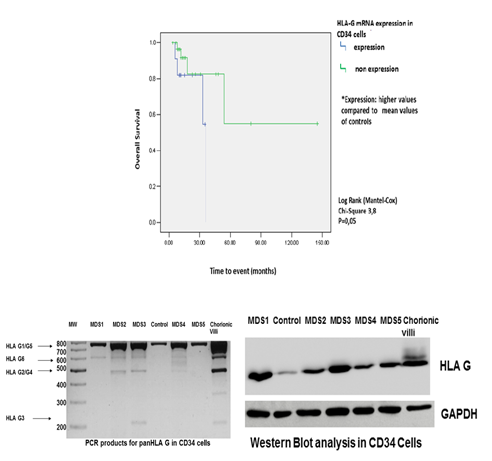
Real Time PCR for HLA-G mRNA expression was performed in CD34 bone marrow (BM) cells derived from 35 primary untreated MDS patients of all subtypes, 7 patients with high-grade Non Hodgkin Lymphoma without BM involvement, as well as first trimester trophoblasts from 2 donors served as controls. CD34 BM HLA-G protein levels were evaluated by Western blot in 10 study participants and plasma HLA-G protein levels were evaluated by ELISA in 22 MDS samples and 17 apparently healthy age/sex matched controls. Canonical variate analysis (CVA) was used to discriminate MDS subtypes using a set of several well established laboratory parameters, as well as the HLA-G expression in CD34+ cells. The Kaplan–Meier method was used for calculation of survival probabilities and the Log-rank test for comparison of survival curves between expression levels of HLA-G. Cox regression was used for Overall Survival (OS).
Results
Increased HLA-G mRNA and protein expression was observed in CD34 cells from MDS patients compared to controls (p=0.04 and p=0.0095, respectively). Plasma HLA-G levels were significantly higher in MDS patients compared to controls (p=0.0008). A distinct pattern of expression of various HLA-G mRNA isoforms was noted between MDS patients and controls. HLA G1/G5 was the commonest isoform expressed in CD34 cells, but other less common isoforms namely HLA-G2/-G4,-G3, and –G6 were also expressed. Interestingly, the CVA revealed that the major parameters that play a role in discrimination of the three major MDS subtypes (RCUD, RCMD and RAEBs), were not only the expected parameters of WPSS and percentage of blasts, but also the HLA-G mRNA expression in CD34 cells. OS curves of MDS with HLA-G mRNA overexpression differed significantly during follow-up, compared to MDS patients without expression (p = 0.05).
Conclusion
Given the immune inhibitory properties of the HLA-G molecule, its increased expression in MDS CD34 cells may implicate the mechanisms of resistance or escape to immune surveillance contributing to MDS evolution and prognosis. Moreover, in the era of demethylating treatment the finding of HLA-G expression by MDS CD34 cells is of specific clinical interest.
Session topic: 9. Myelodysplastic syndromes – Biology & Translational Research
Keyword(s): HLA, Immunity, Myelodysplasia, Prognostic factor
Abstract: PB2075
Type: Publication Only
Background
In myelodysplastic syndromes (MDS) deregulation of immune effectors pathogenetically drives abnormal haemopoiesis and enhanced leukemic propensity. Human leukocyte antigen G (HLA-G) is a nonclassical MHC class I antigen, regularly not expressed in normal tissues except in trophoblasts from early gestation placentas and other immune-privileged tissues. Evidence for HLA-G silencing by a DNA methylation process has been reported. Interestingly, the use of demethylating agents such as 5-azacytidine further demonstrated that the repression of HLA-G gene activity in cultured various cell lines is reversed by demethylating treatment. HLA-G primary transcript generates seven alternative mRNAs that encode membrane-bound HLA-G1, -G2, -G3, -G4 and soluble HLA-G5,-G6 and –G7 isoforms. HLA-G has a direct inhibitory effect on the cytolytic activity of NK cells, cytotoxic T lymphocytes and is implicated in the induction of Foxp3-regulatory T cells. Therefore, HLA-G possesses immune tolerogenic activity with potential implications in antitumor immune responses.
Aims
In order to investigate potential implication of HLA-G in immune deregulation underlying MDS pathogenesis and evolution we studied HLA-G gene and protein expression in CD34 cells of patients with primary MDS.
Methods
Real Time PCR for HLA-G mRNA expression was performed in CD34 bone marrow (BM) cells derived from 35 primary untreated MDS patients of all subtypes, 7 patients with high-grade Non Hodgkin Lymphoma without BM involvement, as well as first trimester trophoblasts from 2 donors served as controls. CD34 BM HLA-G protein levels were evaluated by Western blot in 10 study participants and plasma HLA-G protein levels were evaluated by ELISA in 22 MDS samples and 17 apparently healthy age/sex matched controls. Canonical variate analysis (CVA) was used to discriminate MDS subtypes using a set of several well established laboratory parameters, as well as the HLA-G expression in CD34+ cells. The Kaplan–Meier method was used for calculation of survival probabilities and the Log-rank test for comparison of survival curves between expression levels of HLA-G. Cox regression was used for Overall Survival (OS).
Results
Increased HLA-G mRNA and protein expression was observed in CD34 cells from MDS patients compared to controls (p=0.04 and p=0.0095, respectively). Plasma HLA-G levels were significantly higher in MDS patients compared to controls (p=0.0008). A distinct pattern of expression of various HLA-G mRNA isoforms was noted between MDS patients and controls. HLA G1/G5 was the commonest isoform expressed in CD34 cells, but other less common isoforms namely HLA-G2/-G4,-G3, and –G6 were also expressed. Interestingly, the CVA revealed that the major parameters that play a role in discrimination of the three major MDS subtypes (RCUD, RCMD and RAEBs), were not only the expected parameters of WPSS and percentage of blasts, but also the HLA-G mRNA expression in CD34 cells. OS curves of MDS with HLA-G mRNA overexpression differed significantly during follow-up, compared to MDS patients without expression (p = 0.05).

Conclusion
Given the immune inhibitory properties of the HLA-G molecule, its increased expression in MDS CD34 cells may implicate the mechanisms of resistance or escape to immune surveillance contributing to MDS evolution and prognosis. Moreover, in the era of demethylating treatment the finding of HLA-G expression by MDS CD34 cells is of specific clinical interest.
Session topic: 9. Myelodysplastic syndromes – Biology & Translational Research
Keyword(s): HLA, Immunity, Myelodysplasia, Prognostic factor


