
Contributions
Abstract: PB1917
Type: Publication Only
Background
Two randomized phase 3 trials compared first-line bosutinib vs imatinib in patients (pts) with chronic phase chronic myeloid leukemia (CP CML). In BELA (NCT00574873; 2008–2015), Philadelphia chromosome–positive (Ph+) pts received bosutinib 500 mg once daily (QD) or imatinib 400 mg QD; the primary endpoint was complete cytogenetic response (CCyR) rate at 12 mo. In BFORE (NCT02130557; 2014–ongoing), Ph+ or Ph−/BCR-ABL+ pts received a lower bosutinib starting dose (400 mg QD); the primary endpoint was major molecular response (MMR) rate at 12 mo in the modified intent-to-treat (ITT) population (Ph+ pts with e13a2/e14a2 transcripts).
Aims
Efficacy of bosutinib (400 and 500 mg QD) and imatinib (400 mg QD in both trials) was assessed after ≥12 mo of follow-up from BFORE and BELA, respectively; the safety profile of bosutinib 400 and 500 mg QD was also evaluated.
Methods
Efficacy outcomes were reported for the ITT population, except where noted for the BFORE study. Treatment-emergent adverse events (TEAEs) were assessed in all pts who received ≥1 bosutinib dose, regardless of Ph+ or transcript status.
Results
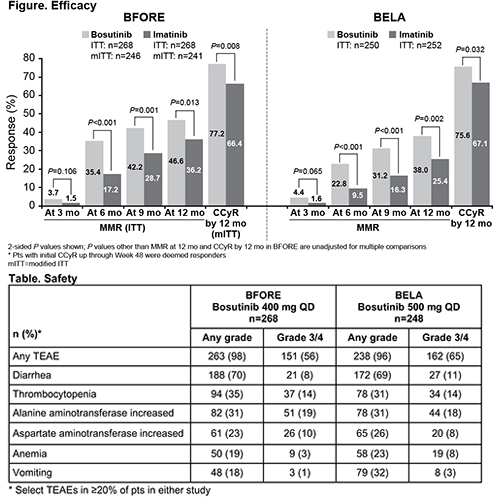
Clinically meaningful and consistent improvements in MMR rates at 6, 9, and 12 mo with bosutinib vs imatinib, as well as in CCyR rate by 12 mo, were seen in BFORE and BELA (Figure). Consistent with the ITT population in BFORE, the MMR rate at the 12-mo visit was higher with bosutinib than imatinib (47.2% vs 36.9%; P=0.02) in the modified ITT population. Despite improved CCyR rate by 12 mo, BELA did not meet the primary endpoint of CCyR rate at 12 mo (bosutinib 70% vs imatinib 68%; P=0.601); in BFORE, CCyR rate at 12 mo was not assessed, as pts with a CCyR but no MMR prior to the 12-mo visit may not have had a cytogenetic assessment at this time point per protocol. Transformation to accelerated/blast phase was similar with bosutinib vs imatinib in BFORE (1.5% vs 2.2%) but less frequent in the bosutinib arm in BELA (1.6% vs 4.8%). Median bosutinib dose intensity (relative dose intensity) was 392 mg/day (98%) in BFORE and 482 mg/day (96%) in BELA; corresponding values for imatinib were 400 mg/day (100%) in both studies. The safety profile of bosutinib was generally similar in BFORE and BELA, except for a lower rate of vomiting (18% and 32%, respectively; Table). No new safety signals were identified for bosutinib in BFORE. Lower percentages of bosutinib-treated pts in BFORE vs BELA had grade 3/4 TEAEs (56% vs 65%; study drug–related 49% vs 57%), serious TEAEs (20% vs 25%), dose delays due to TEAEs (57% vs 63%), and dose reductions due to TEAEs (34% vs 37%). A higher percentage of bosutinib-treated pts remained on study drug after ≥12 mo of follow-up of BFORE vs BELA (78% vs 71%) with lower discontinuation rates due to TEAEs (14% vs 21%).
Conclusion
Increased physician experience in TEAE management with the lower bosutinib starting dose in BFORE vs BELA (400 vs 500 mg QD) likely contributed to the success of BFORE. Despite differences in study endpoints, bosutinib dose, and era of tyrosine kinase inhibitor therapy, the improved efficacy profile with bosutinib vs imatinib in newly diagnosed pts with CP CML was consistent between BFORE and BELA.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Clinical Trial, Tyrosine kinase inhibitor
Abstract: PB1917
Type: Publication Only
Background
Two randomized phase 3 trials compared first-line bosutinib vs imatinib in patients (pts) with chronic phase chronic myeloid leukemia (CP CML). In BELA (NCT00574873; 2008–2015), Philadelphia chromosome–positive (Ph+) pts received bosutinib 500 mg once daily (QD) or imatinib 400 mg QD; the primary endpoint was complete cytogenetic response (CCyR) rate at 12 mo. In BFORE (NCT02130557; 2014–ongoing), Ph+ or Ph−/BCR-ABL+ pts received a lower bosutinib starting dose (400 mg QD); the primary endpoint was major molecular response (MMR) rate at 12 mo in the modified intent-to-treat (ITT) population (Ph+ pts with e13a2/e14a2 transcripts).
Aims
Efficacy of bosutinib (400 and 500 mg QD) and imatinib (400 mg QD in both trials) was assessed after ≥12 mo of follow-up from BFORE and BELA, respectively; the safety profile of bosutinib 400 and 500 mg QD was also evaluated.
Methods
Efficacy outcomes were reported for the ITT population, except where noted for the BFORE study. Treatment-emergent adverse events (TEAEs) were assessed in all pts who received ≥1 bosutinib dose, regardless of Ph+ or transcript status.
Results
Clinically meaningful and consistent improvements in MMR rates at 6, 9, and 12 mo with bosutinib vs imatinib, as well as in CCyR rate by 12 mo, were seen in BFORE and BELA (Figure). Consistent with the ITT population in BFORE, the MMR rate at the 12-mo visit was higher with bosutinib than imatinib (47.2% vs 36.9%; P=0.02) in the modified ITT population. Despite improved CCyR rate by 12 mo, BELA did not meet the primary endpoint of CCyR rate at 12 mo (bosutinib 70% vs imatinib 68%; P=0.601); in BFORE, CCyR rate at 12 mo was not assessed, as pts with a CCyR but no MMR prior to the 12-mo visit may not have had a cytogenetic assessment at this time point per protocol. Transformation to accelerated/blast phase was similar with bosutinib vs imatinib in BFORE (1.5% vs 2.2%) but less frequent in the bosutinib arm in BELA (1.6% vs 4.8%). Median bosutinib dose intensity (relative dose intensity) was 392 mg/day (98%) in BFORE and 482 mg/day (96%) in BELA; corresponding values for imatinib were 400 mg/day (100%) in both studies. The safety profile of bosutinib was generally similar in BFORE and BELA, except for a lower rate of vomiting (18% and 32%, respectively; Table). No new safety signals were identified for bosutinib in BFORE. Lower percentages of bosutinib-treated pts in BFORE vs BELA had grade 3/4 TEAEs (56% vs 65%; study drug–related 49% vs 57%), serious TEAEs (20% vs 25%), dose delays due to TEAEs (57% vs 63%), and dose reductions due to TEAEs (34% vs 37%). A higher percentage of bosutinib-treated pts remained on study drug after ≥12 mo of follow-up of BFORE vs BELA (78% vs 71%) with lower discontinuation rates due to TEAEs (14% vs 21%).

Conclusion
Increased physician experience in TEAE management with the lower bosutinib starting dose in BFORE vs BELA (400 vs 500 mg QD) likely contributed to the success of BFORE. Despite differences in study endpoints, bosutinib dose, and era of tyrosine kinase inhibitor therapy, the improved efficacy profile with bosutinib vs imatinib in newly diagnosed pts with CP CML was consistent between BFORE and BELA.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Clinical Trial, Tyrosine kinase inhibitor


