
Contributions
Abstract: PB1916
Type: Publication Only
Background
Data on survival in CP-CML outside clinical trials are limited. SIMPLICITY (NCT01244750) is an ongoing observational study of CP-CML patients (pts) in routine clinical practice receiving first-line (1L) imatinib (IM), dasatinib (DAS) or nilotinib (NIL) in the US and Europe.
Aims
To describe survival in SIMPLICITY pts with 1, 3 and 5 years of follow-up after start of 1L IM, DAS or NIL.
Methods
Demographics and clinical characteristics are given for pts alive at 5 years and pts who died by 5 years for the overall prospective population and 1L tyrosine kinase inhibitor (TKI) cohorts. Descriptive p-values were generated using Chi-squared tests (categorical variables) and t-tests (continuous variables). Mean survival rate at 1, 3 and 5 years was calculated using the Kaplan-Meier estimator. Mean survival rates are presented for the overall population, for pts who remained on 1L TKI (by 1L TKI) and for pts who switched to 2L TKI (by most recent TKI at follow-up). P-values comparing mean survival rates were based on log-rank test.
Results
By September 07 2017, 1240 pts were enrolled prospectively in SIMPLICITY. Numbers of pts followed for 1, 3 and 5 years were 1192, 1027, and 526 respectively. By Year 3, 50 pts had died, 142 discontinued and 21 pts not reached 3-year follow-up. By Year 5, 78 pts had died, 240 discontinued and 396 pts had yet to reach 5-year follow-up. Median (interquartile range; min–max) age of pts was 55 (47–65; 19–87) years in pts alive at 5 years and 71 (65–78; 19–90) years in pts who had died by 5 years. Other demographics were comparable, but pts alive at 5 years had a mean (±standard deviation) number of comorbidities of 3.2 (±2.6) vs 4.6 (±3.3) in pts who had died by 5 years (p<0.001). Patient characteristics were similar across 1L TKI cohorts. Mean survival rate at 1, 3 and 5 years was 99%, 96%, and 93% for the overall population; 98%, 94%, and 91% for 1L IM pts; 99%, 98%, and 96% for 1L DAS pts; and 99%, 95%, and 91% for 1L NIL pts. Mean 5-year survival rates were higher in 1L DAS pts vs 1L IM or 1L NIL, pending consideration of confounding variables. The 5-year survival rate was higher for pts remaining on 1L DAS vs pts on 2L DAS (97% vs 91%; p=0.02); similarly for pts who remained on 1L NIL vs pts switched from 1L NIL (95% vs 85%; p=0.005). At 5 years 78 pts had died in the overall population over a mean follow-up of 4 years. In the IM, DAS, and NIL cohorts, 36, 13 and 29 pts had died over a mean follow-up of 4–4.25 years. In the overall population, 15 deaths were related to CP-CML, 41 unrelated to CP-CML and 22 had unknown relationship to CP-CML. Of deaths in the IM, DAS, and NIL cohorts, 8, 4 and 3 were related to CP-CML, and 16, 5 and 20 were unrelated to CP-CML.
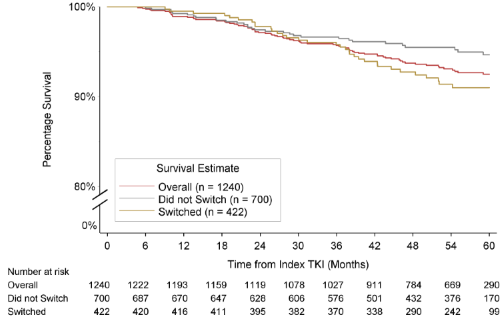
Conclusion
Survival rates in the overall SIMPLICITY population, and in pts receiving IM, DAS, and NIL, are high. Survival rates were higher in pts who remained on 1L DAS and 1L NIL than pts who switched. Survival rates and the proportions of pts who died by 5 years differed between TKI cohorts. Due to the descriptive nature of the statistics, comparisons should be made cautiously. Advanced survival modeling will consider other confounders such as age, gender, etc. These preliminary findings suggest use of TKIs is associated with long-term survival benefit in routine clinical practice. In future analyses with longer median follow-up differences may be discernible between different TKI cohorts and analysis groups.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Survival, Tyrosine kinase inhibitor
Abstract: PB1916
Type: Publication Only
Background
Data on survival in CP-CML outside clinical trials are limited. SIMPLICITY (NCT01244750) is an ongoing observational study of CP-CML patients (pts) in routine clinical practice receiving first-line (1L) imatinib (IM), dasatinib (DAS) or nilotinib (NIL) in the US and Europe.
Aims
To describe survival in SIMPLICITY pts with 1, 3 and 5 years of follow-up after start of 1L IM, DAS or NIL.
Methods
Demographics and clinical characteristics are given for pts alive at 5 years and pts who died by 5 years for the overall prospective population and 1L tyrosine kinase inhibitor (TKI) cohorts. Descriptive p-values were generated using Chi-squared tests (categorical variables) and t-tests (continuous variables). Mean survival rate at 1, 3 and 5 years was calculated using the Kaplan-Meier estimator. Mean survival rates are presented for the overall population, for pts who remained on 1L TKI (by 1L TKI) and for pts who switched to 2L TKI (by most recent TKI at follow-up). P-values comparing mean survival rates were based on log-rank test.
Results
By September 07 2017, 1240 pts were enrolled prospectively in SIMPLICITY. Numbers of pts followed for 1, 3 and 5 years were 1192, 1027, and 526 respectively. By Year 3, 50 pts had died, 142 discontinued and 21 pts not reached 3-year follow-up. By Year 5, 78 pts had died, 240 discontinued and 396 pts had yet to reach 5-year follow-up. Median (interquartile range; min–max) age of pts was 55 (47–65; 19–87) years in pts alive at 5 years and 71 (65–78; 19–90) years in pts who had died by 5 years. Other demographics were comparable, but pts alive at 5 years had a mean (±standard deviation) number of comorbidities of 3.2 (±2.6) vs 4.6 (±3.3) in pts who had died by 5 years (p<0.001). Patient characteristics were similar across 1L TKI cohorts. Mean survival rate at 1, 3 and 5 years was 99%, 96%, and 93% for the overall population; 98%, 94%, and 91% for 1L IM pts; 99%, 98%, and 96% for 1L DAS pts; and 99%, 95%, and 91% for 1L NIL pts. Mean 5-year survival rates were higher in 1L DAS pts vs 1L IM or 1L NIL, pending consideration of confounding variables. The 5-year survival rate was higher for pts remaining on 1L DAS vs pts on 2L DAS (97% vs 91%; p=0.02); similarly for pts who remained on 1L NIL vs pts switched from 1L NIL (95% vs 85%; p=0.005). At 5 years 78 pts had died in the overall population over a mean follow-up of 4 years. In the IM, DAS, and NIL cohorts, 36, 13 and 29 pts had died over a mean follow-up of 4–4.25 years. In the overall population, 15 deaths were related to CP-CML, 41 unrelated to CP-CML and 22 had unknown relationship to CP-CML. Of deaths in the IM, DAS, and NIL cohorts, 8, 4 and 3 were related to CP-CML, and 16, 5 and 20 were unrelated to CP-CML.

Conclusion
Survival rates in the overall SIMPLICITY population, and in pts receiving IM, DAS, and NIL, are high. Survival rates were higher in pts who remained on 1L DAS and 1L NIL than pts who switched. Survival rates and the proportions of pts who died by 5 years differed between TKI cohorts. Due to the descriptive nature of the statistics, comparisons should be made cautiously. Advanced survival modeling will consider other confounders such as age, gender, etc. These preliminary findings suggest use of TKIs is associated with long-term survival benefit in routine clinical practice. In future analyses with longer median follow-up differences may be discernible between different TKI cohorts and analysis groups.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Survival, Tyrosine kinase inhibitor


