
Contributions
Abstract: PB1949
Type: Publication Only
Background
Normally, information regarding efficacy of treatments is obtained through clinical trials which are characterized by their strict inclusion and exclusion criteria.
In real life, results depend on the relationship between the physician and the patient and on the selection of first and subsequent lines of treatment for patients who show intolerance or resistance to TKI treatment.
Aims
TKI treatment description results among TKI patients with CML from the RALMC in real life: Overall survival rates (OS) as it relates with leukemia (LRS), progression and event free survival (PFS and EFS). Rates of discontinuation due to inefficacy and intolerance.
To compare effectiveness and security among imatinib and 2GTKI (nilotinib and dasatinib).
Methods
Retrospective descriptive analysis of patients from the RALMC with clinical and therapeutic follow-up since January 2002 to December 2016.
Analysis of survival rates through Kaplan-Meier method, distinguishing among OS: time span since diagnosis until death due to any cause; LRS: time span since diagnosis until dead due to a cause connected to CML; PFS: time span since diagnosis until progression to AP or BC or death and EFS: time span since diagnosis until death due to any cause, progression of illness or change of treatment on first line due to inefficacy or intolerance.
Results
505 patients, 279 males (55.2%), 104 (20.6%) of whom were over 70 years. 427 (84.6%) began on Imatinib, 46 (9.1%) on Nilotinib, and 32 (6.3%) on Dasatinib.
131 patients (30.7%) with imatinib in first-line changed treatment: 46 (10.8%) due to intolerance and 85 (19.9%) due to inefficacy. 11 patients with 2GTKI (14.1%) changed treatmente too: 6 (7.7%) due to intolerance and 5 (6.4%) due to inefficacy.
21 patients (4.2%) progressed to advanced phases: 14 (2.8%) to BC and 7 (1.4%) to AP.
63 patients (12.5%) died during the follow-up. 15 (23.8%) due to causes related to CML and 48 (76.2%) due to causes not directly related to CML. 49 patients over 70 (47.1%) died during the follow-up: 41 (83.7%) due to causes not related to CML.
Global series | OS | LRS | PFS | EFS |
5 years | 92.9% | 98.3% | 91.2% | 69.7% |
10 years | 85.4% | 97.2% | 82.9% | 57.6% |
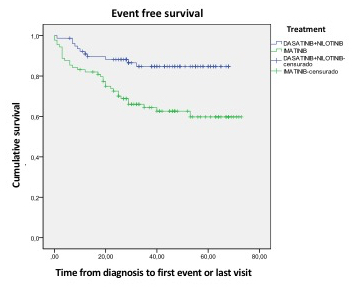
OS, PFS and EFS are higher among patients treated with 2GTKI vs Imatinib to 5 years (98.6% vs 81.6%; 97.2% vs 80.5%; 84.7% vs 59.8%, p=0.002)
Conclusion
Patients from RALMC present high OS rates and death causes are not related directly with the illness, independently of time of diagnosis.
Patients treated on first line with 2GTKI present better OS, PFS and EFS rates than those treated with Imatinib.
While these results are limited in our series: young patients and high risk patients begin their treatment on 2GTKI whereas older and with low-risk prognostication begin their treatment on Imatinib. Mean age for patients treated with Imatinib was almost 10 years older than that of patients treated with 2GTKI, with three times more patients over 70 years old being treated with Imatinib than with 2GTKI (27% vs 10.3%).
Session topic: 8. Chronic myeloid leukemia - Clinical
Abstract: PB1949
Type: Publication Only
Background
Normally, information regarding efficacy of treatments is obtained through clinical trials which are characterized by their strict inclusion and exclusion criteria.
In real life, results depend on the relationship between the physician and the patient and on the selection of first and subsequent lines of treatment for patients who show intolerance or resistance to TKI treatment.
Aims
TKI treatment description results among TKI patients with CML from the RALMC in real life: Overall survival rates (OS) as it relates with leukemia (LRS), progression and event free survival (PFS and EFS). Rates of discontinuation due to inefficacy and intolerance.
To compare effectiveness and security among imatinib and 2GTKI (nilotinib and dasatinib).
Methods
Retrospective descriptive analysis of patients from the RALMC with clinical and therapeutic follow-up since January 2002 to December 2016.
Analysis of survival rates through Kaplan-Meier method, distinguishing among OS: time span since diagnosis until death due to any cause; LRS: time span since diagnosis until dead due to a cause connected to CML; PFS: time span since diagnosis until progression to AP or BC or death and EFS: time span since diagnosis until death due to any cause, progression of illness or change of treatment on first line due to inefficacy or intolerance.
Results
505 patients, 279 males (55.2%), 104 (20.6%) of whom were over 70 years. 427 (84.6%) began on Imatinib, 46 (9.1%) on Nilotinib, and 32 (6.3%) on Dasatinib.
131 patients (30.7%) with imatinib in first-line changed treatment: 46 (10.8%) due to intolerance and 85 (19.9%) due to inefficacy. 11 patients with 2GTKI (14.1%) changed treatmente too: 6 (7.7%) due to intolerance and 5 (6.4%) due to inefficacy.
21 patients (4.2%) progressed to advanced phases: 14 (2.8%) to BC and 7 (1.4%) to AP.
63 patients (12.5%) died during the follow-up. 15 (23.8%) due to causes related to CML and 48 (76.2%) due to causes not directly related to CML. 49 patients over 70 (47.1%) died during the follow-up: 41 (83.7%) due to causes not related to CML.
Global series | OS | LRS | PFS | EFS |
5 years | 92.9% | 98.3% | 91.2% | 69.7% |
10 years | 85.4% | 97.2% | 82.9% | 57.6% |
OS, PFS and EFS are higher among patients treated with 2GTKI vs Imatinib to 5 years (98.6% vs 81.6%; 97.2% vs 80.5%; 84.7% vs 59.8%, p=0.002)

Conclusion
Patients from RALMC present high OS rates and death causes are not related directly with the illness, independently of time of diagnosis.
Patients treated on first line with 2GTKI present better OS, PFS and EFS rates than those treated with Imatinib.
While these results are limited in our series: young patients and high risk patients begin their treatment on 2GTKI whereas older and with low-risk prognostication begin their treatment on Imatinib. Mean age for patients treated with Imatinib was almost 10 years older than that of patients treated with 2GTKI, with three times more patients over 70 years old being treated with Imatinib than with 2GTKI (27% vs 10.3%).
Session topic: 8. Chronic myeloid leukemia - Clinical


