
Contributions
Abstract: PB1926
Type: Publication Only
Background
Although switching to generic imatinib is increasingly used across the world, mainly because of economic reasons, there is a paucity of information regarding the effects of this switch on plasma levels, response and treatment tolerance.
Aims
Primary: Determine and compare plasma levels of imatinib after switching from Glivec to generic imatinib. Secondary: To assess if response or tolerance are changed after switching.
Methods
Imatinib plasma levels, BCR-ABL (IS) values and tolerance were measured before switching (while the patient was taken Glivec), and after 1 month and 3 months of treatment with generic imatinib (all patients took the same brand of generic imatinib).
The study was approved by the Ethics committee, and all patients gave their informed consent. Plasma levels were measured using liquid chromatography tandem mass spectrometry method as previously published by our group. BCR-ABL values are expressed in IS. Cmin, Cmax, BCR-ABL values and standard hematologic and biochemistry values (including PTH and CK values) were entered in the statistic model. Descriptive statistics, means comparison and correlations were done using SPSS package.
Results
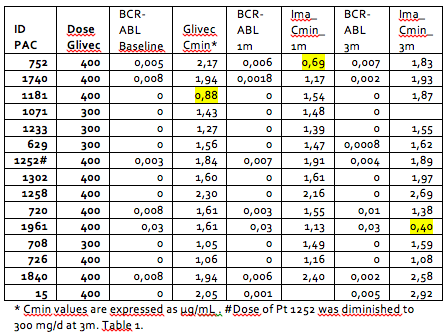
All patients treated in our institution with Glivec were included. As most of our patients with CML are treated upfront with 2G TKI, only 15 patients have been included. The median duration of Glivec treatment before switching was 109 months (11-191). All patients but one (ID:1961) were at MR4 or better at baseline. At baseline, all the patients had adequate Cmin values(≥ 1μg/mL), except one (ID:1181), who has indetectable transcript at that time. After change to generic Imatinib, all samples showed adequate Cmin values, except two (ID:752, +1m and ID 1961, +3m). When comparing baseline with 1 month and 3 month values, there were no significant differences between the mean values of Cmin, BCR-ABL values and standard hematologic values. The only significant difference was between phosphate baseline and 1-month values (3±0.43 vs 2.77±0.27 mg/dl; p=0.039).
As regard to response, all patients with MR4 or better maintained this kind of response, and the same occured with the patient with MMR.
Concerning tolerance, there was no change in adverse events (AE) in any patient. In patient 1252, as plasma levels at baseline, 1m and 3m were higher than 1.8μg/mL, and considering that he had multiple low-grade toxicity, dose was reduced at 3m, with amelioration of AE.
Conclusion
Taking into account the limitation of our study (small sample size), it seems that changing from Glivec to generic Imatinib is not associated with significant changes in Cmin Imatinib values, BCR-ABL values or adverse events. The prompt and simultaneous measurement of plasma levels and molecular response has allowed us to reassure patients about the change, and to adjust the dose in one patient, diminishing the toxicity. Based on these results, we conclude that this approach is useful in patients whose treatment is switched from Glivec to generic imatinib.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Generic drugs, imatinib
Abstract: PB1926
Type: Publication Only
Background
Although switching to generic imatinib is increasingly used across the world, mainly because of economic reasons, there is a paucity of information regarding the effects of this switch on plasma levels, response and treatment tolerance.
Aims
Primary: Determine and compare plasma levels of imatinib after switching from Glivec to generic imatinib. Secondary: To assess if response or tolerance are changed after switching.
Methods
Imatinib plasma levels, BCR-ABL (IS) values and tolerance were measured before switching (while the patient was taken Glivec), and after 1 month and 3 months of treatment with generic imatinib (all patients took the same brand of generic imatinib).
The study was approved by the Ethics committee, and all patients gave their informed consent. Plasma levels were measured using liquid chromatography tandem mass spectrometry method as previously published by our group. BCR-ABL values are expressed in IS. Cmin, Cmax, BCR-ABL values and standard hematologic and biochemistry values (including PTH and CK values) were entered in the statistic model. Descriptive statistics, means comparison and correlations were done using SPSS package.
Results
All patients treated in our institution with Glivec were included. As most of our patients with CML are treated upfront with 2G TKI, only 15 patients have been included. The median duration of Glivec treatment before switching was 109 months (11-191). All patients but one (ID:1961) were at MR4 or better at baseline. At baseline, all the patients had adequate Cmin values(≥ 1μg/mL), except one (ID:1181), who has indetectable transcript at that time. After change to generic Imatinib, all samples showed adequate Cmin values, except two (ID:752, +1m and ID 1961, +3m). When comparing baseline with 1 month and 3 month values, there were no significant differences between the mean values of Cmin, BCR-ABL values and standard hematologic values. The only significant difference was between phosphate baseline and 1-month values (3±0.43 vs 2.77±0.27 mg/dl; p=0.039).
As regard to response, all patients with MR4 or better maintained this kind of response, and the same occured with the patient with MMR.
Concerning tolerance, there was no change in adverse events (AE) in any patient. In patient 1252, as plasma levels at baseline, 1m and 3m were higher than 1.8μg/mL, and considering that he had multiple low-grade toxicity, dose was reduced at 3m, with amelioration of AE.

Conclusion
Taking into account the limitation of our study (small sample size), it seems that changing from Glivec to generic Imatinib is not associated with significant changes in Cmin Imatinib values, BCR-ABL values or adverse events. The prompt and simultaneous measurement of plasma levels and molecular response has allowed us to reassure patients about the change, and to adjust the dose in one patient, diminishing the toxicity. Based on these results, we conclude that this approach is useful in patients whose treatment is switched from Glivec to generic imatinib.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): BCR-ABL, Chronic myeloid leukemia, Generic drugs, imatinib


