
Contributions
Abstract: PB1875
Type: Publication Only
Background
Ibrutinib is a BTK inhibitor, indicated for use in CLL/SLL patients as a second line therapy when purine analogues are either infective, or contraindicated. Standard dosing regime is 420 mg daily. Complications associated with this level of dosing include skin lesions or infections, cardiac arrhythmias, hypertension, bleeding, secondary malignancies and other infections. It has been suggested that reduced dosing may be equally efficacious in this patient group.
Aims
To consider the efficacy of reduced dose of ibrutinib (maintenance dosing) for retaining remission in CLL/SLL patients who have achieved CR or VGPR on full dose therapy.
Methods
A retrospective data audit was conducted on all patients who had received maintenance dosing of ibrutinib at Southern Sydney Haematology. Criteria being assessed included total time on therapy, time on maintenance dosing, effects of reduced dosing on any side effects, evidence of relapse, and basic demographic data.
Results
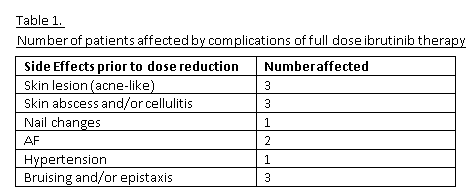
Out of 40 patients treated with ibrutinib at the facility, a total of 13 patients had received reduced dosing with ibrutinib: 8 males and 5 females. Median age was 69 (range 63 – 77). Total time on therapy was a median of 35 months (range 31-36 months) while time on reduced dosing was a median of 14 months (range 6-24 months). Seven patients had developed various side effects on full dose therapy (table 1) but none of these side effects recurred on reduced dosing. One patient developed new onset atrial fibrillation seven months after starting reduced dosing of ibrutinib that is currently medication controlled. No patients have relapsed on reduced dosing and this entire cohort remains on maintenance therapy.
Conclusion
Based on findings from this cohort, reduced dosing of ibrutinib 980 mg -1260 mg/week for patients who have achieved VGPR or CR and have been stable on therapy for 12-24 months would appear to be sufficient to maintain remission and a good way for managing skin lesions or other minor side effects. This confirms the results of other small-scale studies conducted at other institutions.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, Dose intensity, ibrutinib
Abstract: PB1875
Type: Publication Only
Background
Ibrutinib is a BTK inhibitor, indicated for use in CLL/SLL patients as a second line therapy when purine analogues are either infective, or contraindicated. Standard dosing regime is 420 mg daily. Complications associated with this level of dosing include skin lesions or infections, cardiac arrhythmias, hypertension, bleeding, secondary malignancies and other infections. It has been suggested that reduced dosing may be equally efficacious in this patient group.
Aims
To consider the efficacy of reduced dose of ibrutinib (maintenance dosing) for retaining remission in CLL/SLL patients who have achieved CR or VGPR on full dose therapy.
Methods
A retrospective data audit was conducted on all patients who had received maintenance dosing of ibrutinib at Southern Sydney Haematology. Criteria being assessed included total time on therapy, time on maintenance dosing, effects of reduced dosing on any side effects, evidence of relapse, and basic demographic data.
Results
Out of 40 patients treated with ibrutinib at the facility, a total of 13 patients had received reduced dosing with ibrutinib: 8 males and 5 females. Median age was 69 (range 63 – 77). Total time on therapy was a median of 35 months (range 31-36 months) while time on reduced dosing was a median of 14 months (range 6-24 months). Seven patients had developed various side effects on full dose therapy (table 1) but none of these side effects recurred on reduced dosing. One patient developed new onset atrial fibrillation seven months after starting reduced dosing of ibrutinib that is currently medication controlled. No patients have relapsed on reduced dosing and this entire cohort remains on maintenance therapy.

Conclusion
Based on findings from this cohort, reduced dosing of ibrutinib 980 mg -1260 mg/week for patients who have achieved VGPR or CR and have been stable on therapy for 12-24 months would appear to be sufficient to maintain remission and a good way for managing skin lesions or other minor side effects. This confirms the results of other small-scale studies conducted at other institutions.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, Dose intensity, ibrutinib


