
Contributions
Abstract: PB1870
Type: Publication Only
Background
In an era with novel agents, chemoimmunotherapy with rituximab (R) still generally constitutes the cornerstone of first-line chronic lymphocytic leukemia (CLL) treatment. International clinical practice guidelines recommend to repeat first-line chemoimmunotherapy if the treatment-free interval between first- and second-line treatment is over 24 to 36 months. However, the effectiveness of R-based regimens in second-line—with or without previous R-based regimens in first-line—has virtually not been evaluated at the population level.
Aims
The aim of this population-based study was to assess the effectiveness of R-based chemotherapy for the second-line treatment of CLL patients in relation with first-line treatment with or without R-based chemotherapy.
Methods
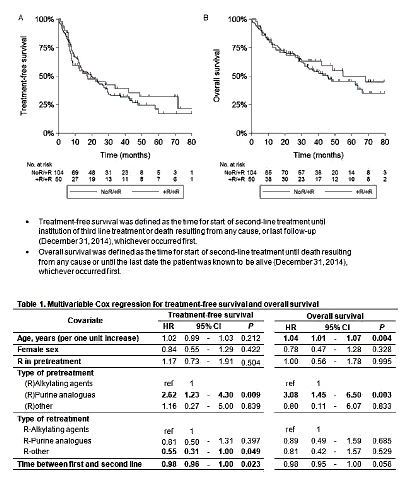
We selected all CLL patients diagnosed between 2004-2008 who initiated second-line treatment with R-containing chemotherapy from the PHAROS CLL registry that encompasses ~40% of the Dutch population and is notified by the nationwide Netherlands Cancer Registry. Patients who initiated second-line treatment with R-based chemotherapy were categorized into two groups, namely those who received first-line chemotherapy with (+R/+R) or without R (NoR/+R). The primary and secondary endpoint was treatment-free survival (TFS) and overall survival (OS), respectively (see definitions in Fig 1). Multivariable Cox regression was used to assess covariates (listed in Table 1) associated with TFS and OS. A P<0.05 indicates statistical significance.
Results
A total of 154 rituximab-treated CLL patients were included in this analysis, of whom 104 (68%) and 50 (32%) were in the NoR/+R and +R/+R group, respectively. Patients in the NoR/+R group were significantly older than patients in the +R/+R group (median age 71 [range 37-88] vs 67 [range 40-83] years; P=0.046). Most patients in the NoR/+R and +R/+R group received first-line treatment with alkylating agents (86% vs 84%; P=0.797), followed by purine analogues (PA; 13% vs 14%; P=0.795). In the second-line setting, 54% and 20% of patients received R with alkylating agents, 22% and 28% with PA, and 24% and 53% with other modalities (i.e. R monotherapy or DHAP) in the NoR/+R and +R/+R group, respectively (P<0.001). At a median follow-up of 30.7 months (range 0.2-115), the median TFS was 19.6 (95% confidence interval [CI], 12.6-27.3) and 17.1 months (95%CI, 7.9-48.6) for the NoR/+R and +R/+R group, respectively (P=0.627). Third-line treatment was started in 48% and 36% and deaths occurred in 51% and 42% in the NoR/+R and +R/+R groups, respectively. The median OS was 45.8 (95% CI, 31.9-66.5) and 54.9 months (95% CI, 27.7-not reached) for the NoR/+R and +R/+R group, respectively (P=0.433). In multivariable analysis, first-line treatment with R ± PA, as compared with R ± alkylating agents, was associated with lower TFS (P=0.009) lower OS (P=0.003). In addition, second-line treatment with R-based regimens without alkylating agents or PA (P=0.049), as compared with R with PA, and time between first- and second-line treatment per one month increase (P=0.023) was associated with better TFS. Furthermore, age per one increase was associated with lower OS (P=0.004).
Conclusion
In this population-based study, we demonstrated that TFS and OS among rituximab-treated CLL patients in second-line was not influenced by first-line chemotherapy with R. This novel finding illustrates that R-based chemotherapy in second-line is a viable treatment option among particular patient subsets in an era with novel, expensive agents.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, Population, Rituximab, Survival
Abstract: PB1870
Type: Publication Only
Background
In an era with novel agents, chemoimmunotherapy with rituximab (R) still generally constitutes the cornerstone of first-line chronic lymphocytic leukemia (CLL) treatment. International clinical practice guidelines recommend to repeat first-line chemoimmunotherapy if the treatment-free interval between first- and second-line treatment is over 24 to 36 months. However, the effectiveness of R-based regimens in second-line—with or without previous R-based regimens in first-line—has virtually not been evaluated at the population level.
Aims
The aim of this population-based study was to assess the effectiveness of R-based chemotherapy for the second-line treatment of CLL patients in relation with first-line treatment with or without R-based chemotherapy.
Methods
We selected all CLL patients diagnosed between 2004-2008 who initiated second-line treatment with R-containing chemotherapy from the PHAROS CLL registry that encompasses ~40% of the Dutch population and is notified by the nationwide Netherlands Cancer Registry. Patients who initiated second-line treatment with R-based chemotherapy were categorized into two groups, namely those who received first-line chemotherapy with (+R/+R) or without R (NoR/+R). The primary and secondary endpoint was treatment-free survival (TFS) and overall survival (OS), respectively (see definitions in Fig 1). Multivariable Cox regression was used to assess covariates (listed in Table 1) associated with TFS and OS. A P<0.05 indicates statistical significance.
Results
A total of 154 rituximab-treated CLL patients were included in this analysis, of whom 104 (68%) and 50 (32%) were in the NoR/+R and +R/+R group, respectively. Patients in the NoR/+R group were significantly older than patients in the +R/+R group (median age 71 [range 37-88] vs 67 [range 40-83] years; P=0.046). Most patients in the NoR/+R and +R/+R group received first-line treatment with alkylating agents (86% vs 84%; P=0.797), followed by purine analogues (PA; 13% vs 14%; P=0.795). In the second-line setting, 54% and 20% of patients received R with alkylating agents, 22% and 28% with PA, and 24% and 53% with other modalities (i.e. R monotherapy or DHAP) in the NoR/+R and +R/+R group, respectively (P<0.001). At a median follow-up of 30.7 months (range 0.2-115), the median TFS was 19.6 (95% confidence interval [CI], 12.6-27.3) and 17.1 months (95%CI, 7.9-48.6) for the NoR/+R and +R/+R group, respectively (P=0.627). Third-line treatment was started in 48% and 36% and deaths occurred in 51% and 42% in the NoR/+R and +R/+R groups, respectively. The median OS was 45.8 (95% CI, 31.9-66.5) and 54.9 months (95% CI, 27.7-not reached) for the NoR/+R and +R/+R group, respectively (P=0.433). In multivariable analysis, first-line treatment with R ± PA, as compared with R ± alkylating agents, was associated with lower TFS (P=0.009) lower OS (P=0.003). In addition, second-line treatment with R-based regimens without alkylating agents or PA (P=0.049), as compared with R with PA, and time between first- and second-line treatment per one month increase (P=0.023) was associated with better TFS. Furthermore, age per one increase was associated with lower OS (P=0.004).

Conclusion
In this population-based study, we demonstrated that TFS and OS among rituximab-treated CLL patients in second-line was not influenced by first-line chemotherapy with R. This novel finding illustrates that R-based chemotherapy in second-line is a viable treatment option among particular patient subsets in an era with novel, expensive agents.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Chronic Lymphocytic Leukemia, Population, Rituximab, Survival


