
Contributions
Abstract: PB1845
Type: Publication Only
Background
Cytomorphologic examination of bone marrow samples from patients with leukemia has been a hallmark of clinical management for decades. More recently, measurable residual disease detection (MRD) has become an important tool for the monitoring of patients with lymphoid malignancies during their course of therapy and for early detection of impending relapse. Conventionally in the USA, bone marrow is collected and analyzed by multiparametric flow cytometry (mpFC) to evaluate MRD status. The sensitivity of mpFC varies but generally is in the range of 10-3 to 10-.5 if one million cells are subjected to analysis. More recent molecular methods such as next-generation sequencing (NGS) allow for more sensitive detection of MRD at 1 in 10-6 if one million cells are analyzed. Thus, for the same number of cells analyzed NGS is approximately 10-100 times more sensitive than flow cytometry. The higher sensitivity of NGS raises the possibility that peripheral blood (PB) samples with an overall lower total tumor burden than bone marrow may nevertheless be suitable for MRD tracking by NGS in certain lymphoid malignancies.
Aims
We conducted a comparative analysis of the levels of MRD by NGS of paired PB and bone marrow samples among patients with lymphoid malignancies in order to determine concordance of MRD between marrow and PB, as well as the sensitivity and specificity of tracking MRD in the PB by NGS.
Methods
Forty-seven paired marrow and PB samples were obtained from 33 patients with lymphoid malignancies: 20 with acute lymphoblastic leukemia (ALL), 11 with chronic lymphocytic leukemia (CLL), and 2 mantle cell lymphoma patients. Genomic DNA was extracted from pre-treatment bone marrow samples and Adaptive Biotechnologies’ NGS MRD assay was used to evaluate the complete B and/or T cell repertoires in order to identify dominant sequences appropriate for MRD tracking. Subsequently, paired PB and bone marrow samples were obtained from this patient cohort and evaluated for MRD via NGS.
Results
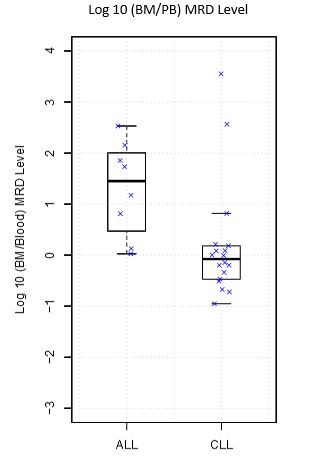
Twenty of 47 paired samples were negative for MRD in both bone marrow and PB. 27 of 47 paired samples were positive for MRD in the bone marrow; 23 of these were also positive for MRD in the corresponding blood sample. When compared to bone marrow, the sensitivity of detection in PB for ALL is 75% and for CLL is 88.9% with a specificity of 100% for both. Comparison of the MRD levels from PB and bone marrow for the 27 MRD positive cases generated a Lin’s concordance coefficient of 0.92. Overall, MRD levels in the marrow were a median of 1.2 times higher than MRD in levels in the PB. Using the criterion of a one hundred fold difference between bone marrow and PB (roughly corresponding to an average difference in sensitivity of mpFC and NGS), 91.5% of the paired samples demonstrated concordance between PB and marrow MRD NGS results. The median ratio of marrow to PB MRD differed significantly between diseases (Marrow:PB MRD of 0.8 in CLL and 28.3 in ALL), Wilcoxon p value = 0.007, see figure. Individual cases with discordant MRD results will be described in detail in the formal presentation.
Conclusion
Monitoring of MRD levels via NGS of the peripheral blood over time may provide an alternative to more invasive bone marrow aspiration and mpFC analysis in patients with lymphoid malignancies. Confirmatory prospective studies of PB MRD monitoring by NGS in patients with lymphoid malignancies are warranted.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): ALL, Chronic Lymphocytic Leukemia, MRD, Peripheral blood
Abstract: PB1845
Type: Publication Only
Background
Cytomorphologic examination of bone marrow samples from patients with leukemia has been a hallmark of clinical management for decades. More recently, measurable residual disease detection (MRD) has become an important tool for the monitoring of patients with lymphoid malignancies during their course of therapy and for early detection of impending relapse. Conventionally in the USA, bone marrow is collected and analyzed by multiparametric flow cytometry (mpFC) to evaluate MRD status. The sensitivity of mpFC varies but generally is in the range of 10-3 to 10-.5 if one million cells are subjected to analysis. More recent molecular methods such as next-generation sequencing (NGS) allow for more sensitive detection of MRD at 1 in 10-6 if one million cells are analyzed. Thus, for the same number of cells analyzed NGS is approximately 10-100 times more sensitive than flow cytometry. The higher sensitivity of NGS raises the possibility that peripheral blood (PB) samples with an overall lower total tumor burden than bone marrow may nevertheless be suitable for MRD tracking by NGS in certain lymphoid malignancies.
Aims
We conducted a comparative analysis of the levels of MRD by NGS of paired PB and bone marrow samples among patients with lymphoid malignancies in order to determine concordance of MRD between marrow and PB, as well as the sensitivity and specificity of tracking MRD in the PB by NGS.
Methods
Forty-seven paired marrow and PB samples were obtained from 33 patients with lymphoid malignancies: 20 with acute lymphoblastic leukemia (ALL), 11 with chronic lymphocytic leukemia (CLL), and 2 mantle cell lymphoma patients. Genomic DNA was extracted from pre-treatment bone marrow samples and Adaptive Biotechnologies’ NGS MRD assay was used to evaluate the complete B and/or T cell repertoires in order to identify dominant sequences appropriate for MRD tracking. Subsequently, paired PB and bone marrow samples were obtained from this patient cohort and evaluated for MRD via NGS.
Results
Twenty of 47 paired samples were negative for MRD in both bone marrow and PB. 27 of 47 paired samples were positive for MRD in the bone marrow; 23 of these were also positive for MRD in the corresponding blood sample. When compared to bone marrow, the sensitivity of detection in PB for ALL is 75% and for CLL is 88.9% with a specificity of 100% for both. Comparison of the MRD levels from PB and bone marrow for the 27 MRD positive cases generated a Lin’s concordance coefficient of 0.92. Overall, MRD levels in the marrow were a median of 1.2 times higher than MRD in levels in the PB. Using the criterion of a one hundred fold difference between bone marrow and PB (roughly corresponding to an average difference in sensitivity of mpFC and NGS), 91.5% of the paired samples demonstrated concordance between PB and marrow MRD NGS results. The median ratio of marrow to PB MRD differed significantly between diseases (Marrow:PB MRD of 0.8 in CLL and 28.3 in ALL), Wilcoxon p value = 0.007, see figure. Individual cases with discordant MRD results will be described in detail in the formal presentation.

Conclusion
Monitoring of MRD levels via NGS of the peripheral blood over time may provide an alternative to more invasive bone marrow aspiration and mpFC analysis in patients with lymphoid malignancies. Confirmatory prospective studies of PB MRD monitoring by NGS in patients with lymphoid malignancies are warranted.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): ALL, Chronic Lymphocytic Leukemia, MRD, Peripheral blood


