
Contributions
Abstract: PB1719
Type: Publication Only
Background
Induction treatments for acute myeloid leukemia (AML) patients are based on the combination of Cytarabine (CYT) and one anthracycline, mainly Idarubicin (IDA), Daunorubicin (DNR), or Mitoxantrone (MIT). The choice of the anthracycline employed has been widely studied in multiple clinical trials showing similar CR rates, with some exceptions in which IDA reported higher CR. A new Personalized Medicine (PM) test developed by Vivia Biotech, based on an actionable native environment method which enables the establishment of pharmacological responses in ex vivo patient samples, is uncovering individual responses to these treatments.
Aims
Our objective is to explore whether a significant percentage of individual patients may respond differently to IDA vs DNR vs MIT treatments, in spite that of their “on average” similar response shown by clinical trials.
Methods
Bone marrow (BM) samples were collected at diagnosis from AML patients. Samples were incubated for 48 hours in 96 well plates, each well containing different drugs or drug combinations at 8 different concentrations, enabling calculation of dose-response (DR) curves for each single drug (CYT, IDA, DNR, MIT) and combination used in treatments (CYT-IDA, CYT-DNR, CYT-MIT). Ex vivo drug sensitivity analysis was made using the PharmaFlow platform maintaining the BM microenvironment. Drug response was evaluated as depletion of AML blast cells in each well after incubation. Annexin V-FITC was used to quantify the ability of the drugs to induce apoptosis, and pharmacological responses were calculated using pharmacokinetic population models.
Results
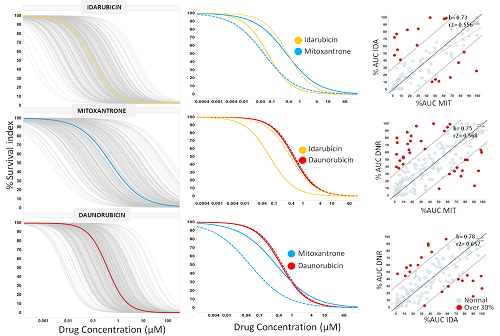
DR graphs were generated for each anthracycline (IDA, DNR and MIT) using PD models. Left panel of figure 1 shows the individual (grey lines) and average DR for IDA (yellow), MIT (blue) and DNR (red) from 289, 274 and 333 AML patients, respectively. As we expected, the average dose responses of the four anthracyclines were similar, with a slight increase in survival index with IDA. However, the interpatient variability of either drug is quite large, which could explain the differences in anthracycline sensitivity reported in some patients. As an example, middle panel figure 1 shows a patient sample that is resistant to IDA and DNR but sensitive to MIT. To identify those cases of selective sensitivity to anthracyclines, we compared the potency, in terms of AUC, between IDA vs. MIT, DNR vs. MIT and DNR vs. IDA (right panel figure 1). Most dots tend to line up, but red dots represent patient samples with a difference in potency between these drugs greater than 30%. Red dots from 3 pairwise comparisons identify 28.3% of patient samples with this different potency between single anthracyclines. Similar DR graphs were generated for CYT-IDA, CYT-DNR and CYT-MIT combinations. The pairwise comparison of these combination treatments obtained different sensitivity in 8.2 % of patients. Overall differences in sensitivity combining both the monotherapies and the CYT combinations were 30.6%.
Conclusion
These results show that this PM test seems able to identify a subset of AML patients whose ex vivo pharmacological response to anthracyclines is significantly different. A fraction of these patients may benefit if treatment selection among these alternatives were to be aided by this PM ex vivo test. To identify which fraction would benefit we would need a trial specifically designed.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Anthracycline, Ara-C (cytarabine), Drug sensitivity
Abstract: PB1719
Type: Publication Only
Background
Induction treatments for acute myeloid leukemia (AML) patients are based on the combination of Cytarabine (CYT) and one anthracycline, mainly Idarubicin (IDA), Daunorubicin (DNR), or Mitoxantrone (MIT). The choice of the anthracycline employed has been widely studied in multiple clinical trials showing similar CR rates, with some exceptions in which IDA reported higher CR. A new Personalized Medicine (PM) test developed by Vivia Biotech, based on an actionable native environment method which enables the establishment of pharmacological responses in ex vivo patient samples, is uncovering individual responses to these treatments.
Aims
Our objective is to explore whether a significant percentage of individual patients may respond differently to IDA vs DNR vs MIT treatments, in spite that of their “on average” similar response shown by clinical trials.
Methods
Bone marrow (BM) samples were collected at diagnosis from AML patients. Samples were incubated for 48 hours in 96 well plates, each well containing different drugs or drug combinations at 8 different concentrations, enabling calculation of dose-response (DR) curves for each single drug (CYT, IDA, DNR, MIT) and combination used in treatments (CYT-IDA, CYT-DNR, CYT-MIT). Ex vivo drug sensitivity analysis was made using the PharmaFlow platform maintaining the BM microenvironment. Drug response was evaluated as depletion of AML blast cells in each well after incubation. Annexin V-FITC was used to quantify the ability of the drugs to induce apoptosis, and pharmacological responses were calculated using pharmacokinetic population models.
Results
DR graphs were generated for each anthracycline (IDA, DNR and MIT) using PD models. Left panel of figure 1 shows the individual (grey lines) and average DR for IDA (yellow), MIT (blue) and DNR (red) from 289, 274 and 333 AML patients, respectively. As we expected, the average dose responses of the four anthracyclines were similar, with a slight increase in survival index with IDA. However, the interpatient variability of either drug is quite large, which could explain the differences in anthracycline sensitivity reported in some patients. As an example, middle panel figure 1 shows a patient sample that is resistant to IDA and DNR but sensitive to MIT. To identify those cases of selective sensitivity to anthracyclines, we compared the potency, in terms of AUC, between IDA vs. MIT, DNR vs. MIT and DNR vs. IDA (right panel figure 1). Most dots tend to line up, but red dots represent patient samples with a difference in potency between these drugs greater than 30%. Red dots from 3 pairwise comparisons identify 28.3% of patient samples with this different potency between single anthracyclines. Similar DR graphs were generated for CYT-IDA, CYT-DNR and CYT-MIT combinations. The pairwise comparison of these combination treatments obtained different sensitivity in 8.2 % of patients. Overall differences in sensitivity combining both the monotherapies and the CYT combinations were 30.6%.

Conclusion
These results show that this PM test seems able to identify a subset of AML patients whose ex vivo pharmacological response to anthracyclines is significantly different. A fraction of these patients may benefit if treatment selection among these alternatives were to be aided by this PM ex vivo test. To identify which fraction would benefit we would need a trial specifically designed.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Anthracycline, Ara-C (cytarabine), Drug sensitivity


