
Contributions
Abstract: PB1734
Type: Publication Only
Background
3+7 chemotherapy (CT) is considered reference treatment for AML patients (pts) treated with a curative intent although the rate of complete remission are lower than those that can be obtained with more intense regimens (eg FLAG-ida).
Early assessment of response in AML by day 14 bone marrow (day 14 BM) has been proposed as a tool to optimize the response rate by the introduction of an early second induction in patients with >10% residual blasts. Recent data and reviews criticize this approach since day 14 BM specificity might be suboptimal and an early re-treatment be too toxic.
In this context we reviewed our experience of an early intensification with FLAG-ida in pts obtaining a suboptimal response at day 14 BM after 3+7.
Aims
To evaluate the safety, the feasibility and the outcome of FLAG-ida as early re-induction for newly diagnosed AML pts treated at our hospital between february 2009 to june 2017.
Methods
Retrospective analysis of 19 consecutive newly diagnosed AML pts who were candidate to early re-induction due to residual blasts > 10% (12-90%) at day 14 BM morphological examination and who were considered fit (without active infection, PS ECOG<2, without renal and/or hepatic impairments) to receive FLAG-ida. As outcomes we defined day 30 and 60 not relapsed mortality, complete remission (CR) rate at day 30 post FLAG-Ida, percentage of patient completing their intention to treat (ITT), event free survival (EFS) and overall survival (OS).
Results
Median age of our series was 60 years (26-78 years). 84% pts were de novoAML. 2 pts were ELN favorable risk, 13 intermediate and 4 adverse. Median follow-up was 669 days (248-1479 days). Median time from the induction start to FLAG-Ida was 17 days (15-23 days).
After FLAG-ida median time to neutrophil recovery was 20 days (16-26 days), we recorded 2 cases of bacterial pneumonia and 2 possible invasive fungal infection which recovered with appropriate therapy.
Non relapse mortality rates at 30 and 60 days from FLAG-ida were 0% and 10.5% (2/19). One pt died in CR for an adenovirus hepatitis, the other for a septic shock (from MDR Pseudomonas aeruginosa) after receiving high dose cytarabine as consolidation.
Seventeen of 19 pts (89.5%) obtained the CR after FLAG-ida (median time 29 days). Two pts (10,5%) were refractory.
Sixteen of these 17 (94%) pts completed treatment according to the ITT: 12 pts received allogeneic stem cell transplantation, 1 pt autologous transplantation and 3 pts consolidation chemotherapy. Twelve pts were alive when we analyzed the data, 9 of them in a durable CR.
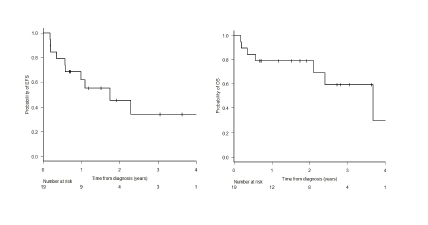
Two year EFS and OS rates were 45.6% (95% CI: 20-68.2) and 78.9% (95% CI: 53.2-91.5) respectively. (Figure 1)
Conclusion
Our findings suggest that FLAG-ida as early reinduction is feasibile and safe at least in a subgroup of AML pts selected on the basis of fitness criteria.
Although recent data raise questions on day 14 BM morphology specificity we believe that our results are of relevance for designing strategies based on early residual disease (eg multi parametric flow cytometry based) assessment in AML to be tested in prospective study.
The number of pts which completed the treatment in respect to the ITT and the persistent CR in spite of the prognostic value of day 14 BM suggest that a strategy of early intensification on the top of 3+7 can be considered in future clinical trials.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AML, Bone Marrow, Induction
Abstract: PB1734
Type: Publication Only
Background
3+7 chemotherapy (CT) is considered reference treatment for AML patients (pts) treated with a curative intent although the rate of complete remission are lower than those that can be obtained with more intense regimens (eg FLAG-ida).
Early assessment of response in AML by day 14 bone marrow (day 14 BM) has been proposed as a tool to optimize the response rate by the introduction of an early second induction in patients with >10% residual blasts. Recent data and reviews criticize this approach since day 14 BM specificity might be suboptimal and an early re-treatment be too toxic.
In this context we reviewed our experience of an early intensification with FLAG-ida in pts obtaining a suboptimal response at day 14 BM after 3+7.
Aims
To evaluate the safety, the feasibility and the outcome of FLAG-ida as early re-induction for newly diagnosed AML pts treated at our hospital between february 2009 to june 2017.
Methods
Retrospective analysis of 19 consecutive newly diagnosed AML pts who were candidate to early re-induction due to residual blasts > 10% (12-90%) at day 14 BM morphological examination and who were considered fit (without active infection, PS ECOG<2, without renal and/or hepatic impairments) to receive FLAG-ida. As outcomes we defined day 30 and 60 not relapsed mortality, complete remission (CR) rate at day 30 post FLAG-Ida, percentage of patient completing their intention to treat (ITT), event free survival (EFS) and overall survival (OS).
Results
Median age of our series was 60 years (26-78 years). 84% pts were de novoAML. 2 pts were ELN favorable risk, 13 intermediate and 4 adverse. Median follow-up was 669 days (248-1479 days). Median time from the induction start to FLAG-Ida was 17 days (15-23 days).
After FLAG-ida median time to neutrophil recovery was 20 days (16-26 days), we recorded 2 cases of bacterial pneumonia and 2 possible invasive fungal infection which recovered with appropriate therapy.
Non relapse mortality rates at 30 and 60 days from FLAG-ida were 0% and 10.5% (2/19). One pt died in CR for an adenovirus hepatitis, the other for a septic shock (from MDR Pseudomonas aeruginosa) after receiving high dose cytarabine as consolidation.
Seventeen of 19 pts (89.5%) obtained the CR after FLAG-ida (median time 29 days). Two pts (10,5%) were refractory.
Sixteen of these 17 (94%) pts completed treatment according to the ITT: 12 pts received allogeneic stem cell transplantation, 1 pt autologous transplantation and 3 pts consolidation chemotherapy. Twelve pts were alive when we analyzed the data, 9 of them in a durable CR.
Two year EFS and OS rates were 45.6% (95% CI: 20-68.2) and 78.9% (95% CI: 53.2-91.5) respectively. (Figure 1)

Conclusion
Our findings suggest that FLAG-ida as early reinduction is feasibile and safe at least in a subgroup of AML pts selected on the basis of fitness criteria.
Although recent data raise questions on day 14 BM morphology specificity we believe that our results are of relevance for designing strategies based on early residual disease (eg multi parametric flow cytometry based) assessment in AML to be tested in prospective study.
The number of pts which completed the treatment in respect to the ITT and the persistent CR in spite of the prognostic value of day 14 BM suggest that a strategy of early intensification on the top of 3+7 can be considered in future clinical trials.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AML, Bone Marrow, Induction


