
Contributions
Abstract: PB1724
Type: Publication Only
Background
Quizartinib (Q) is an oral, highly potent and selective, next-generation FMS-like tyrosine kinase 3 (FLT3) inhibitor. Q has demonstrated promising activity in early clinical studies for treatment of AML and is currently in phase 3 studies for treatment of FLT3-internal tandem duplication (ITD) mutated AML.
Aims
In preclinical studies in rats, food increased oral absorption of Q. Therefore, this study aimed to characterize the effect of a high-fat, high-calorie meal on plasma PK parameters of Q in healthy subjects after a single administration of Q. An additional aim was to evaluate tolerability and safety of Q when administered with or without food.
Methods
Healthy subjects, ages 18-55, were randomized in parallel groups to receive a single 30-mg tablet (formulation used in phase 3 trials) of Q (administered as Q dihydrochloride and equivalent to 26.5 mg free-base) under fasted conditions or after a high-fat, high-calorie meal (fed condition). Plasma levels of Q and its active metabolite, AC886, were measured immediately before Q dosing through 504h post dose using a validated method. PK parameters of Q, AC886, and Q+AC886 were determined using non-compartmental modeling. Ratios (fed/fasted) of geometric least squares means (Geo LSM) and 2-sided 90% confidence intervals (CI) of Cmax, AUClast, and AUCinf of Q were calculated to assess effect of food on PK. A 90%CI entirely within an 80%>125% limit indicated no food effect. Safety and tolerability were assessed.
Results
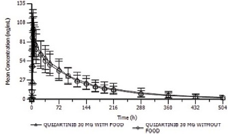
66 subjects were enrolled; 64 received study treatment, 30 under fed conditions and 34 under fasted conditions. Overall, 75% of treated subjects were male and the median age of subjects was 34 years. Mean PK profiles of Q administered under fasted and fed conditions were similar. Administration of Q in the fed condition led to ~8% decrease in Cmax, and increases of ~5% and 8% to AUClast and AUCinf, respectively, vs the fasted condition. The 90%CI for the ratio of the fed versus fasted condition fell within the 80%>125% limits for Cmax and AUClast, and the upper bound of the 90%CI for AUCinf was slightly outside the 125% limit (~128%). Similar trends were also observed for Cmax, AUClast, and AUCinf for AC886 and Q+AC886. Presence of food delayed Tmax of Q by 2h (Tmax was 4h in fasted vs 6h in fed subjects). T1/2 of Q and AC886 was comparable in fasted and fed conditions. All TEAEs reported were mild or moderate; no serious AEs or discontinuations due to AE occurred. Overall, 6 subjects (9.4%) had a TEAE considered related to Q: 2 subjects in the fed condition and 4 subjects in the fasted condition. No significant ECG abnormalities were observed.
Table. Statistical Analysis of Q PK Parameters | ||||
PK Parameter | Fed State | Fasted State | Ratio of Geo LSM | 90% CI for |
Geo LSM (N) | Geo LSM (N) | |||
Cmax, ng/mL | 90.94 (29) | 99.31 (34) | 91.58 | 82.15, 102.08 |
AUClast, ng•h/mL | 8788.07 (29) | 8338.27 (34) | 105.39 | 90.79, 122.35 |
AUCinf, ng•h/mL | 9459.56 (27) | 8727.51 (30) | 108.39 | 91.54, 128.34 |
Conclusion
Administration of food resulted in slight increase in Q exposure, but is not clinically relevant. TEAEs were similar following Q administration in the fed and fasted conditions. Therefore, Q can be administered with or without food.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, AML, Pharmacokinetic, Tyrosine kinase inhibitor
Abstract: PB1724
Type: Publication Only
Background
Quizartinib (Q) is an oral, highly potent and selective, next-generation FMS-like tyrosine kinase 3 (FLT3) inhibitor. Q has demonstrated promising activity in early clinical studies for treatment of AML and is currently in phase 3 studies for treatment of FLT3-internal tandem duplication (ITD) mutated AML.
Aims
In preclinical studies in rats, food increased oral absorption of Q. Therefore, this study aimed to characterize the effect of a high-fat, high-calorie meal on plasma PK parameters of Q in healthy subjects after a single administration of Q. An additional aim was to evaluate tolerability and safety of Q when administered with or without food.
Methods
Healthy subjects, ages 18-55, were randomized in parallel groups to receive a single 30-mg tablet (formulation used in phase 3 trials) of Q (administered as Q dihydrochloride and equivalent to 26.5 mg free-base) under fasted conditions or after a high-fat, high-calorie meal (fed condition). Plasma levels of Q and its active metabolite, AC886, were measured immediately before Q dosing through 504h post dose using a validated method. PK parameters of Q, AC886, and Q+AC886 were determined using non-compartmental modeling. Ratios (fed/fasted) of geometric least squares means (Geo LSM) and 2-sided 90% confidence intervals (CI) of Cmax, AUClast, and AUCinf of Q were calculated to assess effect of food on PK. A 90%CI entirely within an 80%>125% limit indicated no food effect. Safety and tolerability were assessed.
Results
66 subjects were enrolled; 64 received study treatment, 30 under fed conditions and 34 under fasted conditions. Overall, 75% of treated subjects were male and the median age of subjects was 34 years. Mean PK profiles of Q administered under fasted and fed conditions were similar. Administration of Q in the fed condition led to ~8% decrease in Cmax, and increases of ~5% and 8% to AUClast and AUCinf, respectively, vs the fasted condition. The 90%CI for the ratio of the fed versus fasted condition fell within the 80%>125% limits for Cmax and AUClast, and the upper bound of the 90%CI for AUCinf was slightly outside the 125% limit (~128%). Similar trends were also observed for Cmax, AUClast, and AUCinf for AC886 and Q+AC886. Presence of food delayed Tmax of Q by 2h (Tmax was 4h in fasted vs 6h in fed subjects). T1/2 of Q and AC886 was comparable in fasted and fed conditions. All TEAEs reported were mild or moderate; no serious AEs or discontinuations due to AE occurred. Overall, 6 subjects (9.4%) had a TEAE considered related to Q: 2 subjects in the fed condition and 4 subjects in the fasted condition. No significant ECG abnormalities were observed.
Table. Statistical Analysis of Q PK Parameters | ||||
PK Parameter | Fed State | Fasted State | Ratio of Geo LSM | 90% CI for |
Geo LSM (N) | Geo LSM (N) | |||
Cmax, ng/mL | 90.94 (29) | 99.31 (34) | 91.58 | 82.15, 102.08 |
AUClast, ng•h/mL | 8788.07 (29) | 8338.27 (34) | 105.39 | 90.79, 122.35 |
AUCinf, ng•h/mL | 9459.56 (27) | 8727.51 (30) | 108.39 | 91.54, 128.34 |

Conclusion
Administration of food resulted in slight increase in Q exposure, but is not clinically relevant. TEAEs were similar following Q administration in the fed and fasted conditions. Therefore, Q can be administered with or without food.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, AML, Pharmacokinetic, Tyrosine kinase inhibitor


