
Contributions
Abstract: PB1725
Type: Publication Only
Background
Quizartinib is a highly potent, selective FLT3 inhibitor currently being investigated in phase3 studies in patients with AML with FLT3 internal tandem duplication mutations. Acid reducing agents (ARA) such as proton pump inhibitors are frequently used during AML treatment.
Aims
To evaluate the effect of gastric pH on bioavailability of quizartinib, using in vitro data describing pH-dependent solubility profile of quizartinib dihydrochloride as a drug substance, and data from a phase 1 study in healthy volunteers to assess the effect of ARA (the proton pump inhibitor lansoprazole) coadministered with quizartinib as a tablet formulation, on pharmacokinetics (PK) of quizartinib.
Methods
pH-dependent solubility of quizartinib dihydrochloride as a drug substance was evaluated in aqueous buffers at pH 1.1-8.0 at 37°C. Healthy adult subjects were randomized in an open-label, parallel-group study to receive quizartinib alone (single dose of 30 mg quizartinib dihydrochloride tablet formulation [equivalent to 26.5 mg free base]) or lansoprazole + quizartinib (lansoprazole 2 × 30-mg oral delayed-release capsule once daily from Days 1 to 5; single dose of 30 mg quizartinib on Day 5). Plasma concentrations of quizartinib and its active metabolite, AC886, were measured to 504 hours post dose. PK parameters included maximum observed plasma concentration (Cmax) and area under the concentration-time curve to infinity (AUCinf). Effect of lansoprazole on quizartinib PK was assessed by analysis of variance. Safety and tolerability were also assessed.
Results
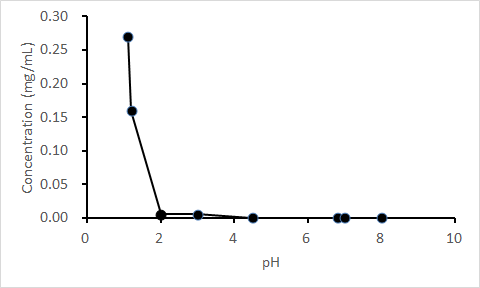
The solubility of quizartinib dihydrochloride as a drug substance decreased sharply with pH ≥2 (Figure). In the phase 1 study (enrolled N = 64), 63 subjects received quizartinib and 59 completed the study. Coadministration of lansoprazole resulted in a lower quizartinib Cmax (~14%) and AUCinf (~5%; Table). The 90% CI for the ratio of AUCinf fell within the 80% to 125% limits. The lower bound of the 90% CI for AUClast and Cmax were slightly below the limit at 79.6% and 78.4%, respectively. However, this decrease is not considered a clinically significant effect. A similar trend was observed with AC886. All treatment-emergent adverse events were mild (n = 19) or moderate (n = 1; headache).
Table. Statistical ANOVA Comparisons of Quizartinib Pharmacokinetic Parameters | ||||||
PK Parameter | Q+L | Q Alone | Ratio of Geo LSM ((Q+L)/Q), % | 90% CI for Ratio of Geo LSM | ||
n | Geo LSM | n | Geo LSM | |||
Cmax, ng/mL | 32 | 90.3 | 30 | 104.8 | 86.11 | 78.36-94.64 |
AUClast, ng•h/mL | 32 | 7825.6 | 30 | 8328.9 | 93.96 | 79.63-110.86 |
AUCinf, ng•h/mL | 31 | 8257.4 | 30 | 8664.7 | 95.30 | 80.16-113.30 |
ANOVA, analysis of variance; CI, confidence interval; Geo LSM, geometric least squares mean; | ||||||
Conclusion
Although quizartinib dihydrochloride demonstrated pH-dependent solubility in vitro as a drug substance, the proton pump inhibitor lansoprazole had no clinically significant effect on quizartinib PK. Quizartinib as a formulated tablet can be coadministered with and without acid reducing agents.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Tyrosine kinase inhibitor, Acute Myeloid Leukemia, Drug interaction, Pharmacokinetic
Abstract: PB1725
Type: Publication Only
Background
Quizartinib is a highly potent, selective FLT3 inhibitor currently being investigated in phase3 studies in patients with AML with FLT3 internal tandem duplication mutations. Acid reducing agents (ARA) such as proton pump inhibitors are frequently used during AML treatment.
Aims
To evaluate the effect of gastric pH on bioavailability of quizartinib, using in vitro data describing pH-dependent solubility profile of quizartinib dihydrochloride as a drug substance, and data from a phase 1 study in healthy volunteers to assess the effect of ARA (the proton pump inhibitor lansoprazole) coadministered with quizartinib as a tablet formulation, on pharmacokinetics (PK) of quizartinib.
Methods
pH-dependent solubility of quizartinib dihydrochloride as a drug substance was evaluated in aqueous buffers at pH 1.1-8.0 at 37°C. Healthy adult subjects were randomized in an open-label, parallel-group study to receive quizartinib alone (single dose of 30 mg quizartinib dihydrochloride tablet formulation [equivalent to 26.5 mg free base]) or lansoprazole + quizartinib (lansoprazole 2 × 30-mg oral delayed-release capsule once daily from Days 1 to 5; single dose of 30 mg quizartinib on Day 5). Plasma concentrations of quizartinib and its active metabolite, AC886, were measured to 504 hours post dose. PK parameters included maximum observed plasma concentration (Cmax) and area under the concentration-time curve to infinity (AUCinf). Effect of lansoprazole on quizartinib PK was assessed by analysis of variance. Safety and tolerability were also assessed.
Results
The solubility of quizartinib dihydrochloride as a drug substance decreased sharply with pH ≥2 (Figure). In the phase 1 study (enrolled N = 64), 63 subjects received quizartinib and 59 completed the study. Coadministration of lansoprazole resulted in a lower quizartinib Cmax (~14%) and AUCinf (~5%; Table). The 90% CI for the ratio of AUCinf fell within the 80% to 125% limits. The lower bound of the 90% CI for AUClast and Cmax were slightly below the limit at 79.6% and 78.4%, respectively. However, this decrease is not considered a clinically significant effect. A similar trend was observed with AC886. All treatment-emergent adverse events were mild (n = 19) or moderate (n = 1; headache).
Table. Statistical ANOVA Comparisons of Quizartinib Pharmacokinetic Parameters | ||||||
PK Parameter | Q+L | Q Alone | Ratio of Geo LSM ((Q+L)/Q), % | 90% CI for Ratio of Geo LSM | ||
n | Geo LSM | n | Geo LSM | |||
Cmax, ng/mL | 32 | 90.3 | 30 | 104.8 | 86.11 | 78.36-94.64 |
AUClast, ng•h/mL | 32 | 7825.6 | 30 | 8328.9 | 93.96 | 79.63-110.86 |
AUCinf, ng•h/mL | 31 | 8257.4 | 30 | 8664.7 | 95.30 | 80.16-113.30 |
ANOVA, analysis of variance; CI, confidence interval; Geo LSM, geometric least squares mean; | ||||||

Conclusion
Although quizartinib dihydrochloride demonstrated pH-dependent solubility in vitro as a drug substance, the proton pump inhibitor lansoprazole had no clinically significant effect on quizartinib PK. Quizartinib as a formulated tablet can be coadministered with and without acid reducing agents.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Tyrosine kinase inhibitor, Acute Myeloid Leukemia, Drug interaction, Pharmacokinetic


