
Contributions
Abstract: PB1731
Type: Publication Only
Background
Allogeneic hematopoietic cell transplantation (allo-HCT) represents the most effective therapy for high risk acute myeloid leukemia (AML) patients. Due to its morbidity and mortality, patients’ selection is crucial. Several prognostic score are used to define the pre-HCT risk for AML patients, however most of them are dated or difficult to be applied in clinical practice.
Aims
To evaluate the clinical outcome of AML pts submitted to allo-HCT.
Methods
Clinical and laboratory data of 101 AML patients submitted to allo-HCT from October 2006 to June 2017 at the Stem Cells Translantation Unit of Spedali Civili Hospital in Brescia were retrospectively collected. A multivariable model was used to weight parameters associated to lower survival post-HCT, in order to build a prognostic score.
Results
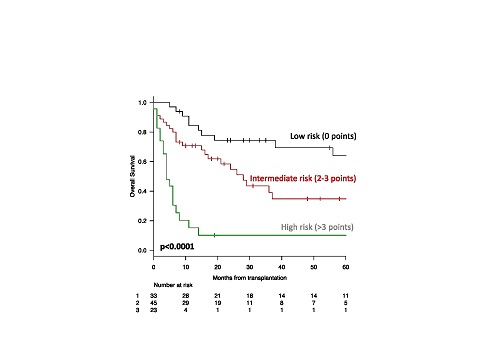
The clinical features of the cohort were the following (median):age 52y (18-67), ≥55y, 47%; male, 56%; AML with myelodysplasia related changes (7%); therapy-related (9%). Karyotype was normal in 39%, unfavorable in 54% and favorable in 7% of cases. Seventeen patients (17%) had FLT3-ITD, 20 (20%) NPM1 and 5 (5%) CEBPA mutations. WT1 gene was overexpressed in 56% of patients. ELN risk category was low/intermediate 1 in 41 (40%), intermediate-2/unfavourable in 60 (60%) patients, respectively.Twenty-one patients (21%)presented chemo-refractoriness to induction treatment; 81(80%) were submitted to allo-HCT in complete hematological remission. Allo-HCT was performed after a median time from diagnosis of 9 months (range, 3-118), conditioning regimen was myeloablative in 55% of cases; matched-related donor was employed in 43 cases (43%), matched-unrelated in 44 (44%), and alternative in 14 (13%) cases. Peripheral blood was the most frequent source of stem cells (73%). According to Sorror HTC-CI, 6 (6%) patients presented 0, 20 (20%) 1, 10 (10%) 2 and 65 (64%) 3 or higher score. Seventy-two patients had a Karnofsky (KPS) equal to 90-100, 29 (28%) pts <90. After a median follow-up of 31 months (range, 6-133) from allo-HCT, acute and chronic graft versus host disease (GVHD) were encountered in 38% and 20% of patients, respectively. At last follow-up, 55 patients (54%) died, for a 2-year overall survival (OS) of 51%. Causes of death were related to: allo-HCT complications (TRM) (30%), disease relapse (66%), other unrelated in 4% of cases, respectively. Among baseline features, age ≥55 years (p=0.03), disease-persistence at transplantation (p<0.001), induction chemotherapy refractoriness (p=0.001), and KPS <90 (p<0.001) significantly correlated with higher mortality. Notably, no differences were observed according to HCT-CI (p=0.096). In multivariate analysis, age ≥55 (HR 2.3 CI95% 1.3-4), disease status at transplantation (HR 2.2 CI95% 1.04-4.8) and lower performance status (HR 2.6 CI95% 1.2-5.4) confirmed their negative prognostic association. Based on these HR, a weighted score was developed; accordingly, age received 2 points, disease-persistence 2 points and KPS 3 points. Low (0 points), intermediate (2-3 points) and high risk (>3 points) categories were so projected to an OS of 74%, 54% and 10% at 2 years, respectively (p<0.0001)(figure 1).
Conclusion
In our experience, advanced disease and performance status were the most important prognostic factors for allo-HTC. With the limitations of the retrospective nature of the study and the number of enrolled patients, our simple clinical score seems to predict allo-HCT outcome in AML patients. Further larger studies are needed to confirm these preliminary data.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Allogeneic hematopoietic stem cell transplant, AML, Prognostic factor
Abstract: PB1731
Type: Publication Only
Background
Allogeneic hematopoietic cell transplantation (allo-HCT) represents the most effective therapy for high risk acute myeloid leukemia (AML) patients. Due to its morbidity and mortality, patients’ selection is crucial. Several prognostic score are used to define the pre-HCT risk for AML patients, however most of them are dated or difficult to be applied in clinical practice.
Aims
To evaluate the clinical outcome of AML pts submitted to allo-HCT.
Methods
Clinical and laboratory data of 101 AML patients submitted to allo-HCT from October 2006 to June 2017 at the Stem Cells Translantation Unit of Spedali Civili Hospital in Brescia were retrospectively collected. A multivariable model was used to weight parameters associated to lower survival post-HCT, in order to build a prognostic score.
Results
The clinical features of the cohort were the following (median):age 52y (18-67), ≥55y, 47%; male, 56%; AML with myelodysplasia related changes (7%); therapy-related (9%). Karyotype was normal in 39%, unfavorable in 54% and favorable in 7% of cases. Seventeen patients (17%) had FLT3-ITD, 20 (20%) NPM1 and 5 (5%) CEBPA mutations. WT1 gene was overexpressed in 56% of patients. ELN risk category was low/intermediate 1 in 41 (40%), intermediate-2/unfavourable in 60 (60%) patients, respectively.Twenty-one patients (21%)presented chemo-refractoriness to induction treatment; 81(80%) were submitted to allo-HCT in complete hematological remission. Allo-HCT was performed after a median time from diagnosis of 9 months (range, 3-118), conditioning regimen was myeloablative in 55% of cases; matched-related donor was employed in 43 cases (43%), matched-unrelated in 44 (44%), and alternative in 14 (13%) cases. Peripheral blood was the most frequent source of stem cells (73%). According to Sorror HTC-CI, 6 (6%) patients presented 0, 20 (20%) 1, 10 (10%) 2 and 65 (64%) 3 or higher score. Seventy-two patients had a Karnofsky (KPS) equal to 90-100, 29 (28%) pts <90. After a median follow-up of 31 months (range, 6-133) from allo-HCT, acute and chronic graft versus host disease (GVHD) were encountered in 38% and 20% of patients, respectively. At last follow-up, 55 patients (54%) died, for a 2-year overall survival (OS) of 51%. Causes of death were related to: allo-HCT complications (TRM) (30%), disease relapse (66%), other unrelated in 4% of cases, respectively. Among baseline features, age ≥55 years (p=0.03), disease-persistence at transplantation (p<0.001), induction chemotherapy refractoriness (p=0.001), and KPS <90 (p<0.001) significantly correlated with higher mortality. Notably, no differences were observed according to HCT-CI (p=0.096). In multivariate analysis, age ≥55 (HR 2.3 CI95% 1.3-4), disease status at transplantation (HR 2.2 CI95% 1.04-4.8) and lower performance status (HR 2.6 CI95% 1.2-5.4) confirmed their negative prognostic association. Based on these HR, a weighted score was developed; accordingly, age received 2 points, disease-persistence 2 points and KPS 3 points. Low (0 points), intermediate (2-3 points) and high risk (>3 points) categories were so projected to an OS of 74%, 54% and 10% at 2 years, respectively (p<0.0001)(figure 1).

Conclusion
In our experience, advanced disease and performance status were the most important prognostic factors for allo-HTC. With the limitations of the retrospective nature of the study and the number of enrolled patients, our simple clinical score seems to predict allo-HCT outcome in AML patients. Further larger studies are needed to confirm these preliminary data.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Allogeneic hematopoietic stem cell transplant, AML, Prognostic factor


