
Contributions
Abstract: PB1723
Type: Publication Only
Background
Enasidenib is an oral, potent inhibitor of mutant IDH2 (mIDH2) proteins. In a phase 1/2 study (NCT01915498) in patients with mIDH2 myeloid malignancies, 38% of patients developed bilirubin elevations, likely due to off-target inhibition of the UGT1A1 enzyme, which metabolizes bilirubin in humans. Homozygous UGT1A1 mutations (UGT1A1mut/mut) are associated with inherited Gilbert Syndrome. A protocol amendment was added to include UGT1A1 gene testing at patient screening.
Aims
To evaluate bilirubin changes associated with UGT1A1 mutational status of patients with mIDH2 hematologic malignancies in the phase 1/2 study of enasidenib monotherapy.
Methods
Enasidenib doses ranged from 50 to 650 mg/day. Total blood bilirubin (TBili) was assessed in all patients at baseline and at each site visit. Normal TBili ranges varied among reference labs (~3-25 µmol/L).
Results
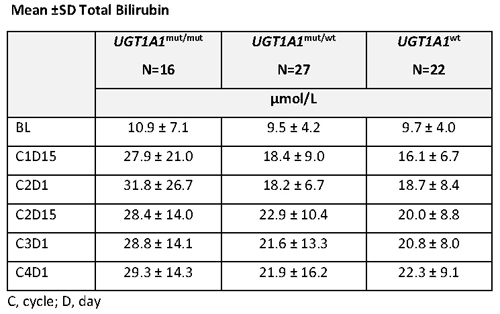
As of Sept 2017, UGT1A1 data were available for 65 patients: 16 patients had homozygous mUGT1A1 (UGT1A1m/m), 27 had heterozygous mUGT1A1 (UGT1A1mut/wt), and 22 had wild type UGT1A1 (UGT1A1wt). 60 patients received enasidenib 100 mg/d and 5 patients (UGT1A1mut/mut n=2; UGT1A1mut/wt n=3) received >100 mg/d enasidenib. Mean TBili at baseline was similar among the 3 genotypes (Table). Patients with UGT1A1mut/mut had more rapid TBili increases during early treatment than the 2 other genotypes, followed by stabilization of TBili levels; mean increases from baseline during treatment in patients with UGT1A1mut/mut indicated mild hyperbilirubinemia (~1.5 x ULN). Mean TBili levels during treatment in patients with UGT1A1mut/wt or UGT1A1wt were within normal range (Table). Indirect bilirubin increases followed similar patterns to those of TBili. AST or ALT increases to ≥3 x ULN during Tx were not more frequent in UGT1A1mut/mut patients (6%) than in UGT1A1mut/wt (15%) or UGT1A1wt (14%) patients. Incidence of treatment-emergent adverse events of hyperbilirubinemia were similar among genotypes although more patients with UGT1A1mut/mut (13%) or UGT1A1mut/wt (11%) had grade 3 bilirubin events than patients with UGT1A1wt (0%). Dose interruptions or reductions due to increased TBili were infrequent in patients with mUGT1A1 and none were reported for UGT1A1wt patients.
Conclusion
These data suggest initial enasidenib dose restrictions are not necessary for patients with UGT1A1 mutations; however, if dose reductions for hyperbilirubinemia are made, they should be informed by the enasidenib prescribing information.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AG-221, Enasidenib, Hyperbilirubinemia, Safety
Abstract: PB1723
Type: Publication Only
Background
Enasidenib is an oral, potent inhibitor of mutant IDH2 (mIDH2) proteins. In a phase 1/2 study (NCT01915498) in patients with mIDH2 myeloid malignancies, 38% of patients developed bilirubin elevations, likely due to off-target inhibition of the UGT1A1 enzyme, which metabolizes bilirubin in humans. Homozygous UGT1A1 mutations (UGT1A1mut/mut) are associated with inherited Gilbert Syndrome. A protocol amendment was added to include UGT1A1 gene testing at patient screening.
Aims
To evaluate bilirubin changes associated with UGT1A1 mutational status of patients with mIDH2 hematologic malignancies in the phase 1/2 study of enasidenib monotherapy.
Methods
Enasidenib doses ranged from 50 to 650 mg/day. Total blood bilirubin (TBili) was assessed in all patients at baseline and at each site visit. Normal TBili ranges varied among reference labs (~3-25 µmol/L).
Results
As of Sept 2017, UGT1A1 data were available for 65 patients: 16 patients had homozygous mUGT1A1 (UGT1A1m/m), 27 had heterozygous mUGT1A1 (UGT1A1mut/wt), and 22 had wild type UGT1A1 (UGT1A1wt). 60 patients received enasidenib 100 mg/d and 5 patients (UGT1A1mut/mut n=2; UGT1A1mut/wt n=3) received >100 mg/d enasidenib. Mean TBili at baseline was similar among the 3 genotypes (Table). Patients with UGT1A1mut/mut had more rapid TBili increases during early treatment than the 2 other genotypes, followed by stabilization of TBili levels; mean increases from baseline during treatment in patients with UGT1A1mut/mut indicated mild hyperbilirubinemia (~1.5 x ULN). Mean TBili levels during treatment in patients with UGT1A1mut/wt or UGT1A1wt were within normal range (Table). Indirect bilirubin increases followed similar patterns to those of TBili. AST or ALT increases to ≥3 x ULN during Tx were not more frequent in UGT1A1mut/mut patients (6%) than in UGT1A1mut/wt (15%) or UGT1A1wt (14%) patients. Incidence of treatment-emergent adverse events of hyperbilirubinemia were similar among genotypes although more patients with UGT1A1mut/mut (13%) or UGT1A1mut/wt (11%) had grade 3 bilirubin events than patients with UGT1A1wt (0%). Dose interruptions or reductions due to increased TBili were infrequent in patients with mUGT1A1 and none were reported for UGT1A1wt patients.

Conclusion
These data suggest initial enasidenib dose restrictions are not necessary for patients with UGT1A1 mutations; however, if dose reductions for hyperbilirubinemia are made, they should be informed by the enasidenib prescribing information.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AG-221, Enasidenib, Hyperbilirubinemia, Safety


