
Contributions
Abstract: S1572
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 08:30 - 08:45
Location: Room A6
Background
Chronic lymphocytic leukemia (CLL) is characterized by proliferation and accumulation of malignant B cells, where this process is associated with constitutively activated B cell receptor (BCR) signaling, and interference with BCR signaling provides therapeutic benefit. Specifically, the Bruton’s Tyrosine Kinase (BTK) inhibitor ibrutinib prevents BTK tyrosine phosphorylation and thereby interferes with the pathway. It has shown high clinical response rates in patients with relapsed and refractory CLL, including patients with adverse cytogenetic profiles.
Despite high responses achieved by ibrutinib, it has important limitations such as inducing CLL cell redistribution from protected niches to the periphery, and the clinical response to ibrutinib is slow and often incomplete. Further, there is no evidence that a cure can be achieved, even among patients that tolerate long-term treatment with ibrutinib, a considerable percentage develops drug resistance, BTK independent disease progression, or Richter’s transformation, indicating drug synergies with ibrutinib may increase prognosis.
Recent studies have explored combined use of ibrutinib with inhibitors for the proteasome (carfilzomib), BCL2i (venetoclax), and HDAC (abexinostat) in preclinical models, which has shown promising initial results. However, these approaches were largely empirical, and do not have systamatic rational.
Aims
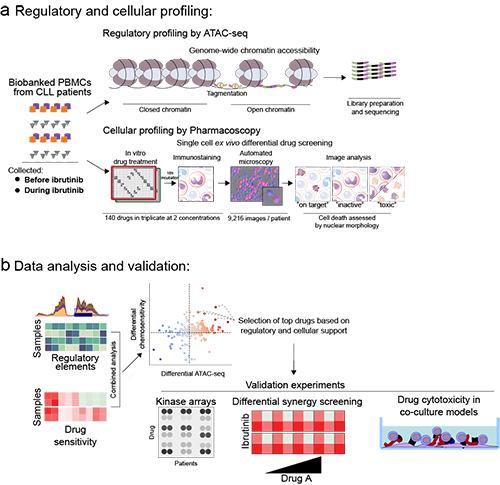
We charted the ibrutinib-induced chromatin regulatory landscape of CLL, and in parallel mapped targetable pathways for synergistic combination therapies that could potentially improve disease control. Peripheral blood from 24 fully characterized CLL cases were collected before and during therapy with ibrutinib. Chromatin accessibility was measured by ATAC-seq to gather the genome-wide regulatory landscape, and ex vivo chemosensitivity to >140 drugs on paired CLL samples was measured using Pharmacoscopy, a translatable method for ex vivo single-cell drug cytotoxicity profiling in primary samples (Snijder et al.Lancet Haematology, 2017;NCT03096821).
Methods
We bioinformatically integrated these datasets, establishing a comprehensive picture of the cellular responses to ibrutinib, and to prioritize targets, we performed secondary differential synergy screening of 21 drugs in combination with Ibrutinib in 8 CLL patient samples taken prior to clinical Ibrutinib treatment, with the goal to visualize changes in the targeted sensitivity of key drugs with and without Ibrutinib treatment.
Results
ATACseq identified increases in chromatin accessibility and chemosensitivity in proteasome, inflammatory NF-κB/TNF signaling, CoA biosynthesis, PI3K/Akt, pathways, along with changes affecting genes such as FOXO3 and IκBa, and both the ex vivo drug testing of samples from patients after clinical ibrutinb and ex vivo synergy screening revealed that ibrutinib treatment in vivo and in vitro sensitizes CLL cells to compounds such as the proteasome inhibitor bortezomib, the JAK inhibitor ruxolitinib, the bisphosphate zoledronate, and the aurora kinase inhibitor ZM447439.
Conclusion
Our results show that synergistic combination and informatics-integration of chromatin profiling with functional drug screening is a powerful tool to identify targetable pathways in CLL. This approach may be useful for designing personalized therapies as well as providing planning for clinical studies. Our approach is directly transferable to other leukemia where malignant cells can be obtained for chromatin profiling and drug sensitivity analysis, thus providing a widely applicable tool for the rational development of combination therapies.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): Drug sensitivity, Ex vivo, ibrutinib, Synergy
Abstract: S1572
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 08:30 - 08:45
Location: Room A6
Background
Chronic lymphocytic leukemia (CLL) is characterized by proliferation and accumulation of malignant B cells, where this process is associated with constitutively activated B cell receptor (BCR) signaling, and interference with BCR signaling provides therapeutic benefit. Specifically, the Bruton’s Tyrosine Kinase (BTK) inhibitor ibrutinib prevents BTK tyrosine phosphorylation and thereby interferes with the pathway. It has shown high clinical response rates in patients with relapsed and refractory CLL, including patients with adverse cytogenetic profiles.
Despite high responses achieved by ibrutinib, it has important limitations such as inducing CLL cell redistribution from protected niches to the periphery, and the clinical response to ibrutinib is slow and often incomplete. Further, there is no evidence that a cure can be achieved, even among patients that tolerate long-term treatment with ibrutinib, a considerable percentage develops drug resistance, BTK independent disease progression, or Richter’s transformation, indicating drug synergies with ibrutinib may increase prognosis.
Recent studies have explored combined use of ibrutinib with inhibitors for the proteasome (carfilzomib), BCL2i (venetoclax), and HDAC (abexinostat) in preclinical models, which has shown promising initial results. However, these approaches were largely empirical, and do not have systamatic rational.
Aims
We charted the ibrutinib-induced chromatin regulatory landscape of CLL, and in parallel mapped targetable pathways for synergistic combination therapies that could potentially improve disease control. Peripheral blood from 24 fully characterized CLL cases were collected before and during therapy with ibrutinib. Chromatin accessibility was measured by ATAC-seq to gather the genome-wide regulatory landscape, and ex vivo chemosensitivity to >140 drugs on paired CLL samples was measured using Pharmacoscopy, a translatable method for ex vivo single-cell drug cytotoxicity profiling in primary samples (Snijder et al.Lancet Haematology, 2017;NCT03096821).
Methods
We bioinformatically integrated these datasets, establishing a comprehensive picture of the cellular responses to ibrutinib, and to prioritize targets, we performed secondary differential synergy screening of 21 drugs in combination with Ibrutinib in 8 CLL patient samples taken prior to clinical Ibrutinib treatment, with the goal to visualize changes in the targeted sensitivity of key drugs with and without Ibrutinib treatment.
Results
ATACseq identified increases in chromatin accessibility and chemosensitivity in proteasome, inflammatory NF-κB/TNF signaling, CoA biosynthesis, PI3K/Akt, pathways, along with changes affecting genes such as FOXO3 and IκBa, and both the ex vivo drug testing of samples from patients after clinical ibrutinb and ex vivo synergy screening revealed that ibrutinib treatment in vivo and in vitro sensitizes CLL cells to compounds such as the proteasome inhibitor bortezomib, the JAK inhibitor ruxolitinib, the bisphosphate zoledronate, and the aurora kinase inhibitor ZM447439.

Conclusion
Our results show that synergistic combination and informatics-integration of chromatin profiling with functional drug screening is a powerful tool to identify targetable pathways in CLL. This approach may be useful for designing personalized therapies as well as providing planning for clinical studies. Our approach is directly transferable to other leukemia where malignant cells can be obtained for chromatin profiling and drug sensitivity analysis, thus providing a widely applicable tool for the rational development of combination therapies.
Session topic: 5. Chronic lymphocytic leukemia and related disorders – Biology & Translational Research
Keyword(s): Drug sensitivity, Ex vivo, ibrutinib, Synergy


