
Contributions
Abstract: S137
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:15 - 12:30
Location: Room A8
Background
Acute leukemia is a heterogeneous malignancy characterized by the clonal expansion of hematopoietic blasts in the peripheral blood, bone marrow, and/or other tissues. A majority of acute leukemia patients have poor prognosis with traditional therapies. T cell therapy, especially with chimeric antigen receptor T (CAR-T) cells, is promising for the treatment of leukemia and other cancers. However, in the context of persistent antigen exposure in chronic viral infections and cancer, both native and adoptive T cells can become exhausted/dysfunctional. Cancer cells can generate large membrane-enclosed structures, known as microvesicles (MVs). Our previous work has demonstrated that BCR-ABL1+ MVs can induce malignant transformation of mononuclear cells from bone marrow.
Aims
The aim of this study was to investigate whether MVs can play a role in leukemia associated T cell exhaustion.
Methods
T cells isolated from healthy donor peripheral were incubated with leukemia-derived MVs. The immune checkpoints inhibitors and function of T cells were examined to explicit the effect of MVs on T cell in vitro. In addition, leukemia-derived MVs were injected into BALB/c mice to detect the effect of MVs on T cells in vivo. The transcriptomes RNA-seq of T cells on day 0, 3 and 7 after incubation with MVs was conducted to excavate the mechanism of T cell exhaustion. Furthermore, bone marrow of leukemia patients were collection to isolated CD3+ T cells through microbeads, then the isolated T cells were cultured in vitro to examined the change of immune checkpoint inhibitors on day 0 and 7.
Results
Following incubation with MVs from various sources, all T cell subtypes exhibited the exhaustion phonotype and impaired cytokine secretion in vitro. Mice models also showed the connection between immune checkpoint inhibitors and MV injection. Sequencing and bioinformatics analyses indicated that a number of transcription factors and microRNAs (miRNAs) were attributable to the dysregulation of pathways and exhaustion in T cells. Further work revealed that functional miR-92a-3p, miR-21-5p, miR-16-5p, miR-126 and miR-182-5p in MVs could be delivered into T cells to induce the exhaustion phenotype. SerpinB2, IL-1β and CXCL5, which are mediators of the NF-κB pathway, were identified as the targets of the miRNAs mentioned above.Interestingly, the immune checkpoint inhibitors of T cells isolated from leukemia patients reduced after culture in vitro, which show that the exhaustion of T cells might be reversed once T cells were separated from tumor environment.
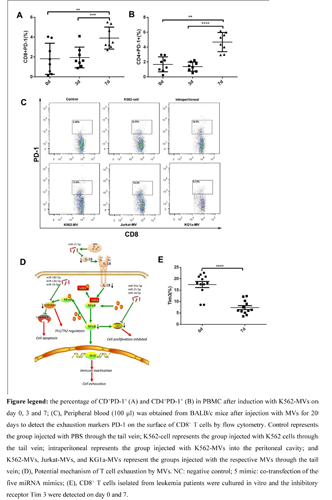
Conclusion
We demonstrated that leukemia-derived MVs could initiate T cell exhaustion via the progressive temporal delivery of multiple exogenous miRNAs into T cells and the subsequent interaction of these miRNAs with their targets. Therefore, MVs can be expected not only to become new indicators of the T cell status in patients but also to be used as novel targets for personalized patient treatment.
Session topic: 25. Gene therapy, cellular immunotherapy and vaccination – Biology & Translational Research
Keyword(s): Microvesicles, T cell depletion
Abstract: S137
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:15 - 12:30
Location: Room A8
Background
Acute leukemia is a heterogeneous malignancy characterized by the clonal expansion of hematopoietic blasts in the peripheral blood, bone marrow, and/or other tissues. A majority of acute leukemia patients have poor prognosis with traditional therapies. T cell therapy, especially with chimeric antigen receptor T (CAR-T) cells, is promising for the treatment of leukemia and other cancers. However, in the context of persistent antigen exposure in chronic viral infections and cancer, both native and adoptive T cells can become exhausted/dysfunctional. Cancer cells can generate large membrane-enclosed structures, known as microvesicles (MVs). Our previous work has demonstrated that BCR-ABL1+ MVs can induce malignant transformation of mononuclear cells from bone marrow.
Aims
The aim of this study was to investigate whether MVs can play a role in leukemia associated T cell exhaustion.
Methods
T cells isolated from healthy donor peripheral were incubated with leukemia-derived MVs. The immune checkpoints inhibitors and function of T cells were examined to explicit the effect of MVs on T cell in vitro. In addition, leukemia-derived MVs were injected into BALB/c mice to detect the effect of MVs on T cells in vivo. The transcriptomes RNA-seq of T cells on day 0, 3 and 7 after incubation with MVs was conducted to excavate the mechanism of T cell exhaustion. Furthermore, bone marrow of leukemia patients were collection to isolated CD3+ T cells through microbeads, then the isolated T cells were cultured in vitro to examined the change of immune checkpoint inhibitors on day 0 and 7.
Results
Following incubation with MVs from various sources, all T cell subtypes exhibited the exhaustion phonotype and impaired cytokine secretion in vitro. Mice models also showed the connection between immune checkpoint inhibitors and MV injection. Sequencing and bioinformatics analyses indicated that a number of transcription factors and microRNAs (miRNAs) were attributable to the dysregulation of pathways and exhaustion in T cells. Further work revealed that functional miR-92a-3p, miR-21-5p, miR-16-5p, miR-126 and miR-182-5p in MVs could be delivered into T cells to induce the exhaustion phenotype. SerpinB2, IL-1β and CXCL5, which are mediators of the NF-κB pathway, were identified as the targets of the miRNAs mentioned above.Interestingly, the immune checkpoint inhibitors of T cells isolated from leukemia patients reduced after culture in vitro, which show that the exhaustion of T cells might be reversed once T cells were separated from tumor environment.

Conclusion
We demonstrated that leukemia-derived MVs could initiate T cell exhaustion via the progressive temporal delivery of multiple exogenous miRNAs into T cells and the subsequent interaction of these miRNAs with their targets. Therefore, MVs can be expected not only to become new indicators of the T cell status in patients but also to be used as novel targets for personalized patient treatment.
Session topic: 25. Gene therapy, cellular immunotherapy and vaccination – Biology & Translational Research
Keyword(s): Microvesicles, T cell depletion


