
Contributions
Abstract: S129
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:30 - 11:45
Location: Room A7
Background
A recent model using clinical-molecular features of patients with secondary myelofibrosis (sMF) was developed by MYSEC (MYelofibrosis SECondary to PV and ET project) showing better prediction of outcome compared with the International Prognostic Scoring System (IPSS); and although the dynamic IPSS (DIPSS) is validated for primary myelofibrosis but also currently used for risk stratification in sMF patients receiving transplant, evaluations of the actual performance of these scores are lacking.
Aims
We aimed to validate and compare the MYSEC and DIPSS in sMF patients undergoing allogeneic stem-cell transplantation.
Methods
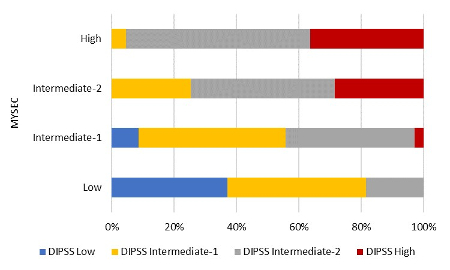
We identified 159 sMF patients who received stem-cell transplant from related (n = 59) or unrelated donors (n = 100) between 2007 and 2015 with available data on blood levels at transplant and presence of mutations at diagnosis. The MYSEC model was calculated as follows: one point was assigned to presence of constitutional symptoms and platelets <150 x109/L. Two points were assigned to hemoglobin <11 g/dl, circulating blasts ≥3% and a CALR-unmutated genotype, whereas 0.15 points were assigned for each year of age. Risk groups (number of patients) according to MYSEC and DIPSS were: low (n = 27 and n = 16), intermediate-1 (n = 70 and n = 59), intermediate-2 (n = 40 and n = 70), and high (n = 22 and n = 14). Scores were validated using Kaplan-Meier estimates while C-statistics were applied to evaluate their discriminatory power.
Results
Median follow-up was 41 months (range, 29 to 52) while the median time between diagnosis and transplant was 128 months (range, 2 to 526). The median age of sMF patients was 52 years (range, 32 to 75). Overall survival at three years was 55.9% (95% confidence interval [CI], 47.5 to 64.3). By stratifying sMF according to evolution from either polycythemia vera (post-PV) or essential thrombocythemia (post-ET), no difference was found in survival at three years being 57.7% (95% CI, 45.5 to 70.0) for post-PV and 54.6% (95% CI, 43.0 to 66.2; p = 0.92) for post-ET.
Overall survival rates at three years according to each risk group of the DIPSS were as follows: 79.5% (95% CI, 58.5 to 100) for the low-risk, 56.3% (95% CI, 42.6 to 70.0) for the intermediate-1-risk, 53.9% (95% CI, 41.2 to 66.6) for the intermediate-2-risk, and 40.8% (95% CI, 14.1 to 67.5) for the high-risk group. Overall, DIPSS was not predictive of outcome (p = 0.30). Regarding MYSEC, probabilities of survival at three years was 69.3% (95% CI, 51.5 to 87.1) for the low-risk, 64.5% (95% CI, 52.5 to 76.5) for the intermediate-1-risk, 46.8 (95% CI, 29.7 to 63.9) for the intermediate-2-risk, and 21.7% (95% CI, 0 to 45.4) for the high-risk group. The MYSEC model was predictive of survival overall (p = 0.03).
When used to assign patients to the four discrete risk categories, the test retained moderate predictability for MYSEC (C-index = 0.585). Prognostic ability was improved in comparison with DIPSS (C-index = 0.546) leading to a significant re-classification of patients (p < 0.001). Due to a considerable difference in age distribution between our transplant cohort and the originally published MYSEC cohort, the score was adjusted by age as continuous variable which even increased the performance of the score showing C-statistics of 0.627.
Conclusion
In comparison to DIPSS, the clinical-molecular system by MYSEC provides a significant re-classification of patients leading to improved prognostic capability in sMF after transplantation. Furthermore, transplant-specific age-adjustment of the MYSEC resulted in an even better predictive power.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): prognosis, Transplant, Myelofibrosis
Abstract: S129
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:30 - 11:45
Location: Room A7
Background
A recent model using clinical-molecular features of patients with secondary myelofibrosis (sMF) was developed by MYSEC (MYelofibrosis SECondary to PV and ET project) showing better prediction of outcome compared with the International Prognostic Scoring System (IPSS); and although the dynamic IPSS (DIPSS) is validated for primary myelofibrosis but also currently used for risk stratification in sMF patients receiving transplant, evaluations of the actual performance of these scores are lacking.
Aims
We aimed to validate and compare the MYSEC and DIPSS in sMF patients undergoing allogeneic stem-cell transplantation.
Methods
We identified 159 sMF patients who received stem-cell transplant from related (n = 59) or unrelated donors (n = 100) between 2007 and 2015 with available data on blood levels at transplant and presence of mutations at diagnosis. The MYSEC model was calculated as follows: one point was assigned to presence of constitutional symptoms and platelets <150 x109/L. Two points were assigned to hemoglobin <11 g/dl, circulating blasts ≥3% and a CALR-unmutated genotype, whereas 0.15 points were assigned for each year of age. Risk groups (number of patients) according to MYSEC and DIPSS were: low (n = 27 and n = 16), intermediate-1 (n = 70 and n = 59), intermediate-2 (n = 40 and n = 70), and high (n = 22 and n = 14). Scores were validated using Kaplan-Meier estimates while C-statistics were applied to evaluate their discriminatory power.
Results
Median follow-up was 41 months (range, 29 to 52) while the median time between diagnosis and transplant was 128 months (range, 2 to 526). The median age of sMF patients was 52 years (range, 32 to 75). Overall survival at three years was 55.9% (95% confidence interval [CI], 47.5 to 64.3). By stratifying sMF according to evolution from either polycythemia vera (post-PV) or essential thrombocythemia (post-ET), no difference was found in survival at three years being 57.7% (95% CI, 45.5 to 70.0) for post-PV and 54.6% (95% CI, 43.0 to 66.2; p = 0.92) for post-ET.
Overall survival rates at three years according to each risk group of the DIPSS were as follows: 79.5% (95% CI, 58.5 to 100) for the low-risk, 56.3% (95% CI, 42.6 to 70.0) for the intermediate-1-risk, 53.9% (95% CI, 41.2 to 66.6) for the intermediate-2-risk, and 40.8% (95% CI, 14.1 to 67.5) for the high-risk group. Overall, DIPSS was not predictive of outcome (p = 0.30). Regarding MYSEC, probabilities of survival at three years was 69.3% (95% CI, 51.5 to 87.1) for the low-risk, 64.5% (95% CI, 52.5 to 76.5) for the intermediate-1-risk, 46.8 (95% CI, 29.7 to 63.9) for the intermediate-2-risk, and 21.7% (95% CI, 0 to 45.4) for the high-risk group. The MYSEC model was predictive of survival overall (p = 0.03).
When used to assign patients to the four discrete risk categories, the test retained moderate predictability for MYSEC (C-index = 0.585). Prognostic ability was improved in comparison with DIPSS (C-index = 0.546) leading to a significant re-classification of patients (p < 0.001). Due to a considerable difference in age distribution between our transplant cohort and the originally published MYSEC cohort, the score was adjusted by age as continuous variable which even increased the performance of the score showing C-statistics of 0.627.

Conclusion
In comparison to DIPSS, the clinical-molecular system by MYSEC provides a significant re-classification of patients leading to improved prognostic capability in sMF after transplantation. Furthermore, transplant-specific age-adjustment of the MYSEC resulted in an even better predictive power.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): prognosis, Transplant, Myelofibrosis


