
Contributions
Abstract: S111
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:45 - 12:00
Location: Room A2
Background
Vitamin D (25-OHD, VD) deficiency has been associated with a poor prognosis in a wide range of cancers. For Hodgkin lymphoma (HL), no data on pretreatment VD levels and their correlation with patient characteristics and outcome exists. Biologically, VD deficiency might render the characteristic T-cell rich microenvironment of HL more tumor-supportive due to a diminished anti-tumor T-cell response (von Essen, 2010).
Aims
To test the hypothesis that pretreatment VD deficiency reduces progression-free survival (PFS) and overall survival (OS) in HL.
Methods
Between 1998 and 2003, the GHSG prospectively recruited 2653 patients of all stages in the GHSG trials HD7, HD8 and HD9. The enriched analysis cohort included all patients with available pretreatment serum sample and documented progression or relapse (N=118), as well as two relapse-free patients each matched by trial and treatment arm (N= 236). VD was measured with a commercially available ELISA. Serum VD levels were categorized according to the Institute of Medicine, Food and Nutrition board (IOM) guidelines defining < 30nmol/l as deficient, 30 to 50 nmol/l as insufficient and ≥ 50nmol/l as sufficient. VD levels and their correlation with other baseline characteristics and outcomes were analyzed descriptively and by linear or logistic regression where applicable. PFS and OS were analyzed with Kaplan-Meier methods and Cox regression; analyses were weighted to attain event rates of the total study cohort. All statistical tests were stratified by trial and treatment arm to account for the matched structure of the analysis cohort.
Results
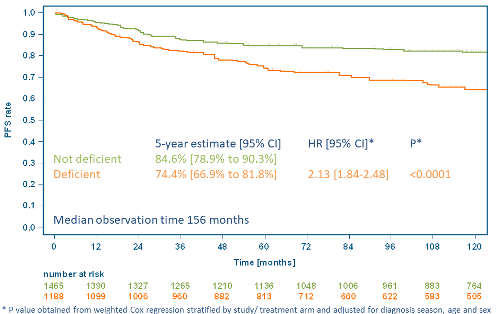
VD levels could be quantified in 351/354 patients in the analysis cohort. VD levels did not correlate with age, sex, clinical stage, a large mediastinal mass, extranodal involvement, presence of 3 or more nodal areas, elevated ESR, B-symptoms, Karnofsky index, IPS, trial or treatment arm and thus were independent of tumor mass, the patients’ clinical condition and the received treatment. VD levels were highly dependent on the season of diagnosis with median levels of 27.7, 42.9, 30.8 and 22.0 nmol/l for spring, summer, autumn and winter, respectively. Thus, according to the IOM cutoffs, 55%, 34%, 48% and 55% of patients diagnosed in spring, summer, autumn and winter, respectively, were deficient (p<0.0001 for summer vs. other). Patients with progression or relapse had a lower median VD level than relapse-free patients (21.4 vs. 35.5 nmol/l) and were more often deficient (68% vs. 41%, p<0.0001). Similar trends were observed across all treatment arms. With a median observation time of 156 months, a lower 5-year PFS rate in deficient patients compared to non-deficient patients was observed (74.4% [95%>CI: 66.9-81.8] vs. 84.6% [95%>CI: 78.9-90.3]). In a multivariate Cox regression adjusted for season of diagnosis, age and sex, VD deficiency was associated with higher risk for a PFS event (HR: 2.13 [95%>CI: 1.84-2.48], p<0.0001). This difference also translated into a clinically significant OS difference with VD deficiency associated with a higher risk for death in a similarly adjusted Cox regression (HR: 1.82 [1.53-2.15], p<0.0001). More deaths in the VD deficient group were HL-related than in the non-deficient group.
Conclusion
Pretreatment VD is a modifiable, independent baseline risk factor for poorer outcome of HL in the present analysis. Supplementation with VD is cheap, generally safe and could potentially improve outcomes in HL patients. Interventional, ideally randomized studies should evaluate VD supplementation as an add-on treatment in HL.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): Hodgkin's disease, Hodgkin's Lymphoma
Abstract: S111
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:45 - 12:00
Location: Room A2
Background
Vitamin D (25-OHD, VD) deficiency has been associated with a poor prognosis in a wide range of cancers. For Hodgkin lymphoma (HL), no data on pretreatment VD levels and their correlation with patient characteristics and outcome exists. Biologically, VD deficiency might render the characteristic T-cell rich microenvironment of HL more tumor-supportive due to a diminished anti-tumor T-cell response (von Essen, 2010).
Aims
To test the hypothesis that pretreatment VD deficiency reduces progression-free survival (PFS) and overall survival (OS) in HL.
Methods
Between 1998 and 2003, the GHSG prospectively recruited 2653 patients of all stages in the GHSG trials HD7, HD8 and HD9. The enriched analysis cohort included all patients with available pretreatment serum sample and documented progression or relapse (N=118), as well as two relapse-free patients each matched by trial and treatment arm (N= 236). VD was measured with a commercially available ELISA. Serum VD levels were categorized according to the Institute of Medicine, Food and Nutrition board (IOM) guidelines defining < 30nmol/l as deficient, 30 to 50 nmol/l as insufficient and ≥ 50nmol/l as sufficient. VD levels and their correlation with other baseline characteristics and outcomes were analyzed descriptively and by linear or logistic regression where applicable. PFS and OS were analyzed with Kaplan-Meier methods and Cox regression; analyses were weighted to attain event rates of the total study cohort. All statistical tests were stratified by trial and treatment arm to account for the matched structure of the analysis cohort.
Results
VD levels could be quantified in 351/354 patients in the analysis cohort. VD levels did not correlate with age, sex, clinical stage, a large mediastinal mass, extranodal involvement, presence of 3 or more nodal areas, elevated ESR, B-symptoms, Karnofsky index, IPS, trial or treatment arm and thus were independent of tumor mass, the patients’ clinical condition and the received treatment. VD levels were highly dependent on the season of diagnosis with median levels of 27.7, 42.9, 30.8 and 22.0 nmol/l for spring, summer, autumn and winter, respectively. Thus, according to the IOM cutoffs, 55%, 34%, 48% and 55% of patients diagnosed in spring, summer, autumn and winter, respectively, were deficient (p<0.0001 for summer vs. other). Patients with progression or relapse had a lower median VD level than relapse-free patients (21.4 vs. 35.5 nmol/l) and were more often deficient (68% vs. 41%, p<0.0001). Similar trends were observed across all treatment arms. With a median observation time of 156 months, a lower 5-year PFS rate in deficient patients compared to non-deficient patients was observed (74.4% [95%>CI: 66.9-81.8] vs. 84.6% [95%>CI: 78.9-90.3]). In a multivariate Cox regression adjusted for season of diagnosis, age and sex, VD deficiency was associated with higher risk for a PFS event (HR: 2.13 [95%>CI: 1.84-2.48], p<0.0001). This difference also translated into a clinically significant OS difference with VD deficiency associated with a higher risk for death in a similarly adjusted Cox regression (HR: 1.82 [1.53-2.15], p<0.0001). More deaths in the VD deficient group were HL-related than in the non-deficient group.

Conclusion
Pretreatment VD is a modifiable, independent baseline risk factor for poorer outcome of HL in the present analysis. Supplementation with VD is cheap, generally safe and could potentially improve outcomes in HL patients. Interventional, ideally randomized studies should evaluate VD supplementation as an add-on treatment in HL.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): Hodgkin's disease, Hodgkin's Lymphoma


