
Contributions
Abstract: S828
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 11:30 - 11:45
Location: Room A7
Background
Exciting responses are reported with Programmed Death 1 inhibitors (PD1i) in Classical Hodgkin Lymphoma (CHL). It is widely believed that PD1i act by reversing CD8 T cell exhaustion, however, predictors of PD1i response in CHL are elusive. Neither CD8 infiltration, PD1 expression nor lymphoma MHC1 expression are predictive as might be expected with a CD8-dependent mechanism of action (MOA). Instead, lymphoma MHC2 expression is associated with response and Programmed Death Ligand 1 (PDL1)-expressing tumor associated macrophages (TAM) interact primarily with PD1+CD4+ not CD8+ cells. These data suggest that CD4+T cells are central to the activity of PD1i in CHL and may be key to identifying better predictors of response.
Aims
To evaluate T cell subsets in CHL and their relationship to the PD1-PDL1 axis.
Methods
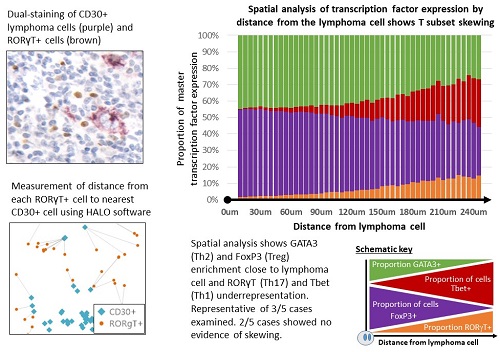
We assessed 146 patients with CHL and 24 reactive lymph node (RLN) controls. Immunohistochemistry (IHC) and novel phenotyping and spatial analysis methods were used to assess TAM, T subsets, lymphoma cells and the PD1 axis. Phenotyping was performed using IHC followed by stripping and re-staining of the same section, allowing accurate assessment of dual-positivity. Spatial analysis was performed using dual-staining on serial sections. CD4+T subset frequency by distance from the lymphoma cell was determined using HALO analysis software. To assess lymphoma influence on TAM, monocyte-derived macrophages (MdMφ) were differentiated from healthy PBMCs and treated with KMH2 CHL cell line supernatant.
Results
CD8+ lymphocytes were frequent, but PD1+ tumour-infiltrating lymphocytes (TIL) were absent or seen at low levels in CHL (median 33% vs 0.08% of nucleated cells). No correlation was seen between PD1+ TIL and PDL1. The absence of a significant PD1+ CD8 population or correlation to PDL1 is inconsistent with a central role for CD8 exhaustion.
Expression of the Th17 transcription factor RORγT was reduced in CHL compared to RLN (p=0.001). Having identified that Th17 suppression was seen in CHL we assessed the role of Th17-suppressing factors; PDL1, Gal1 and IDO1 were upregulated on lymphoma cells and TAM. PDL1+TAM were enriched in areas proximal to lymphoma cells and MdMφ treatment with CHL cell line supernatant led to PDL1 upregulation (p=0.009) suggesting that TAM PDL1 expression is lymphoma induced. We observed an inverse correlation between RORγT+ TIL and PDL1 (p=<0.001) but no correlation between RORγT+ TIL and IDO1 or Gal1. No correlation was seen between PDL1 and other CD4+T-related transcription factors: Tbet (Th1), GATA3 (Th2) or FoxP3 (Treg). Spatial analysis revealed skewing of the T cell infiltrate by distance from the lymphoma cell. Skewed cases revealed enrichment of FoxP3+ and GATA3+ and underrepresentation of Tbet+ and RORγT+ TIL around lymphoma cells (Image, p=<0.0001). This powerful method corrects for variations in tumour content and highlights patterns that are hard to assess by eye. The finding of a distribution similar to Th1 suggests a Th17 anti-tumour role.
Conclusion
The data presented support a model of lymphoma suppression of Th17 cells via the PD1-PDL1 axis. This would explain PD1i activity despite a lack of observed PD1 expression. This is supported by: i) low Th17 numbers, ii) lymphoma-induced expression of Th17-suppressing factors, iii) an inverse correlation to PDL1 expression that is not seen with other CD4+ subsets and iv) a spatial distribution pattern similar to Tbet+ TIL. These data in conjunction with reports of an association between MHC2 with a response to PD1 inhibition highlight a central role for Th17 cells in the MOA of PD1i in CHL.
Session topic: 18. Hodgkin lymphoma – Biology & Translational Research
Keyword(s): Cancer immunotherapy, Hodgkin's disease, Hodgkin's Lymphoma, T lymphocyte, helper
Abstract: S828
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 11:30 - 11:45
Location: Room A7
Background
Exciting responses are reported with Programmed Death 1 inhibitors (PD1i) in Classical Hodgkin Lymphoma (CHL). It is widely believed that PD1i act by reversing CD8 T cell exhaustion, however, predictors of PD1i response in CHL are elusive. Neither CD8 infiltration, PD1 expression nor lymphoma MHC1 expression are predictive as might be expected with a CD8-dependent mechanism of action (MOA). Instead, lymphoma MHC2 expression is associated with response and Programmed Death Ligand 1 (PDL1)-expressing tumor associated macrophages (TAM) interact primarily with PD1+CD4+ not CD8+ cells. These data suggest that CD4+T cells are central to the activity of PD1i in CHL and may be key to identifying better predictors of response.
Aims
To evaluate T cell subsets in CHL and their relationship to the PD1-PDL1 axis.
Methods
We assessed 146 patients with CHL and 24 reactive lymph node (RLN) controls. Immunohistochemistry (IHC) and novel phenotyping and spatial analysis methods were used to assess TAM, T subsets, lymphoma cells and the PD1 axis. Phenotyping was performed using IHC followed by stripping and re-staining of the same section, allowing accurate assessment of dual-positivity. Spatial analysis was performed using dual-staining on serial sections. CD4+T subset frequency by distance from the lymphoma cell was determined using HALO analysis software. To assess lymphoma influence on TAM, monocyte-derived macrophages (MdMφ) were differentiated from healthy PBMCs and treated with KMH2 CHL cell line supernatant.
Results
CD8+ lymphocytes were frequent, but PD1+ tumour-infiltrating lymphocytes (TIL) were absent or seen at low levels in CHL (median 33% vs 0.08% of nucleated cells). No correlation was seen between PD1+ TIL and PDL1. The absence of a significant PD1+ CD8 population or correlation to PDL1 is inconsistent with a central role for CD8 exhaustion.
Expression of the Th17 transcription factor RORγT was reduced in CHL compared to RLN (p=0.001). Having identified that Th17 suppression was seen in CHL we assessed the role of Th17-suppressing factors; PDL1, Gal1 and IDO1 were upregulated on lymphoma cells and TAM. PDL1+TAM were enriched in areas proximal to lymphoma cells and MdMφ treatment with CHL cell line supernatant led to PDL1 upregulation (p=0.009) suggesting that TAM PDL1 expression is lymphoma induced. We observed an inverse correlation between RORγT+ TIL and PDL1 (p=<0.001) but no correlation between RORγT+ TIL and IDO1 or Gal1. No correlation was seen between PDL1 and other CD4+T-related transcription factors: Tbet (Th1), GATA3 (Th2) or FoxP3 (Treg). Spatial analysis revealed skewing of the T cell infiltrate by distance from the lymphoma cell. Skewed cases revealed enrichment of FoxP3+ and GATA3+ and underrepresentation of Tbet+ and RORγT+ TIL around lymphoma cells (Image, p=<0.0001). This powerful method corrects for variations in tumour content and highlights patterns that are hard to assess by eye. The finding of a distribution similar to Th1 suggests a Th17 anti-tumour role.

Conclusion
The data presented support a model of lymphoma suppression of Th17 cells via the PD1-PDL1 axis. This would explain PD1i activity despite a lack of observed PD1 expression. This is supported by: i) low Th17 numbers, ii) lymphoma-induced expression of Th17-suppressing factors, iii) an inverse correlation to PDL1 expression that is not seen with other CD4+ subsets and iv) a spatial distribution pattern similar to Tbet+ TIL. These data in conjunction with reports of an association between MHC2 with a response to PD1 inhibition highlight a central role for Th17 cells in the MOA of PD1i in CHL.
Session topic: 18. Hodgkin lymphoma – Biology & Translational Research
Keyword(s): Cancer immunotherapy, Hodgkin's disease, Hodgkin's Lymphoma, T lymphocyte, helper


