
Contributions
Abstract: S821
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:15 - 12:30
Location: Room K1
Background
Mesenchymal stromal cells (MSCs) exhibit therapeutic activity in steroid-resistant acute Graft versus Host Disease (aGvHD). However, MSC efficacy is completely unpredictable.
Aims
We investigated the fate of infused MSCs and used our findings to design an assay to predict clinical responses
Methods
MSCs were used in a mouse model of GvHD where the disease was mediated by CD8+ cells from Matahari mice (GvHD group) or defective cytotoxic CD8+ cells from Matahari/perforin-/- mice (GvHD perf-/- group). Apoptosis in infused MSCs was evaluated 1 hour after infusion and infiltration of GvHD effector cells in spleen and lungs was assessed 4 days after. We then examined the cytotoxic activity against MSCs of Peripheral Blood Mononuclear Cells (PBMCs) obtained from 31 steroid-resistant aGvHD patients treated with MSCs. When performing the assay, the operator was blind to patients’ clinical details. Informed consent was obtained from all patients in accordance with the local ethics committee requirements. Finally, MSCs made apoptotic in vitro (apoMSCs) before the infusion were tested for their ability to induce immunosuppression in our GvHD mouse model
Results
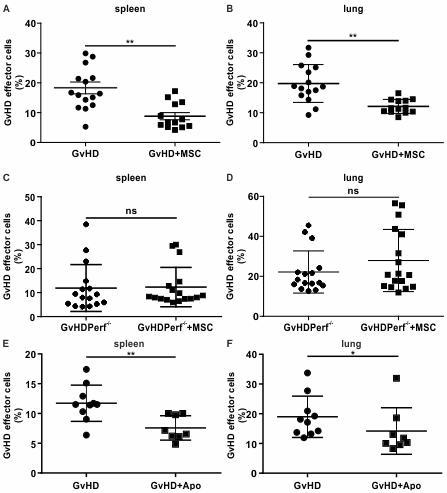
After infusion, we found that MSCs were induced to undergo apoptosis within the first hour but only in the presence of functional cytotoxic GvHD effector cells: mean caspase activation in MSCs was 26.1±7.4 and 6.2±5.6 in GvHD mice and GvHD perf-/- mice, respectively (p=0.0006). Notably, when we assessed the immunosuppressive activity of MSCs, we found that MSCs were able to reduce the infiltration of GvHD effector cells in lungs and spleen only in the GvHD group (Fig. 1 A-D). Taken together, our data demonstrate that cytotoxic cells are required to induce MSC apoptosis which is instrumental for the delivery of immunosuppression. Therefore, we inferred that the presence of cytotoxic cells in the recipient could be predictive of MSC therapeutic activity. PBMCs from 31 patients with steroid-resistant GvHD collected the day before MSC treatment were co-cultured with MSCs and MSC apoptosis was assessed using Annexin V and 7-AAD after 4 hours. The clinical overall response rate to MSCs was 37.5%. PBMCs from responders exhibited a significantly higher cytotoxicity against MSCs in comparison to non-responders (mean: 21.9%±15.4% versus 6.9%±4.7%, respectively (p<.0001) and a cut-off of 11.5% was predictive of response with 91.7% sensitivity and 90.0% specificity. The importance of the cytotoxic assay in predicting the response and its impact on the overall survival (OS) was then assessed. Higher cytotoxicity (>11.5%) was significantly associated with the response in multivariate logistic regression analysis (p<.001) and the median OS was longer in responders than in non-responders: median survival times were not reached and 60.5 days, respectively (log-rank test, p<.03). Finally, to further confirm the role played by MSC apoptosis, we tested the immunosuppressive activity of apoMSCs and we found that the infiltration of GvHD effector cells was significantly reduced both in lungs and spleen after apoMSC infusion (Fig. 1 E, F)
Conclusion
We propose the innovative concept that the presence of functional cytotoxic cells is required to induce MSC apoptosis which in turn is necessary to deliver immunosuppression. Therefore, patients should be stratified for MSC treatment according to their ability to kill MSCs. Alternatively, all patients may be treated with ex vivo apoptotic MSCs
Session topic: 22. Stem cell transplantation - Experimental
Keyword(s): Acute graft-versus-host disease, Apoptosis, Mesenchymal stem cell, Prediction
Abstract: S821
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:15 - 12:30
Location: Room K1
Background
Mesenchymal stromal cells (MSCs) exhibit therapeutic activity in steroid-resistant acute Graft versus Host Disease (aGvHD). However, MSC efficacy is completely unpredictable.
Aims
We investigated the fate of infused MSCs and used our findings to design an assay to predict clinical responses
Methods
MSCs were used in a mouse model of GvHD where the disease was mediated by CD8+ cells from Matahari mice (GvHD group) or defective cytotoxic CD8+ cells from Matahari/perforin-/- mice (GvHD perf-/- group). Apoptosis in infused MSCs was evaluated 1 hour after infusion and infiltration of GvHD effector cells in spleen and lungs was assessed 4 days after. We then examined the cytotoxic activity against MSCs of Peripheral Blood Mononuclear Cells (PBMCs) obtained from 31 steroid-resistant aGvHD patients treated with MSCs. When performing the assay, the operator was blind to patients’ clinical details. Informed consent was obtained from all patients in accordance with the local ethics committee requirements. Finally, MSCs made apoptotic in vitro (apoMSCs) before the infusion were tested for their ability to induce immunosuppression in our GvHD mouse model
Results
After infusion, we found that MSCs were induced to undergo apoptosis within the first hour but only in the presence of functional cytotoxic GvHD effector cells: mean caspase activation in MSCs was 26.1±7.4 and 6.2±5.6 in GvHD mice and GvHD perf-/- mice, respectively (p=0.0006). Notably, when we assessed the immunosuppressive activity of MSCs, we found that MSCs were able to reduce the infiltration of GvHD effector cells in lungs and spleen only in the GvHD group (Fig. 1 A-D). Taken together, our data demonstrate that cytotoxic cells are required to induce MSC apoptosis which is instrumental for the delivery of immunosuppression. Therefore, we inferred that the presence of cytotoxic cells in the recipient could be predictive of MSC therapeutic activity. PBMCs from 31 patients with steroid-resistant GvHD collected the day before MSC treatment were co-cultured with MSCs and MSC apoptosis was assessed using Annexin V and 7-AAD after 4 hours. The clinical overall response rate to MSCs was 37.5%. PBMCs from responders exhibited a significantly higher cytotoxicity against MSCs in comparison to non-responders (mean: 21.9%±15.4% versus 6.9%±4.7%, respectively (p<.0001) and a cut-off of 11.5% was predictive of response with 91.7% sensitivity and 90.0% specificity. The importance of the cytotoxic assay in predicting the response and its impact on the overall survival (OS) was then assessed. Higher cytotoxicity (>11.5%) was significantly associated with the response in multivariate logistic regression analysis (p<.001) and the median OS was longer in responders than in non-responders: median survival times were not reached and 60.5 days, respectively (log-rank test, p<.03). Finally, to further confirm the role played by MSC apoptosis, we tested the immunosuppressive activity of apoMSCs and we found that the infiltration of GvHD effector cells was significantly reduced both in lungs and spleen after apoMSC infusion (Fig. 1 E, F)

Conclusion
We propose the innovative concept that the presence of functional cytotoxic cells is required to induce MSC apoptosis which in turn is necessary to deliver immunosuppression. Therefore, patients should be stratified for MSC treatment according to their ability to kill MSCs. Alternatively, all patients may be treated with ex vivo apoptotic MSCs
Session topic: 22. Stem cell transplantation - Experimental
Keyword(s): Acute graft-versus-host disease, Apoptosis, Mesenchymal stem cell, Prediction


