
Contributions
Abstract: S813
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:30 - 12:45
Location: Room A2
Background
Mastocytosis is a clonal mast cell disease which, in adults, most commonly affects skin, bone marrow and other organs. Symptoms and serum tryptase levels vary, and there are patients with pure cutaneous mastocytosis (CM). It is generally appreciated that all adult patients with MIS require a bone marrow biopsy (BMB) in order to confirm the presence of SM. Although BMB is safe, it may be painful and cause complications. Existing risk scores are based on relatively few patients and a limited set of parameters.
Aims
We aimed at creating a simple and solid risk score to predict SM in adult patients with MIS. We hypothesized that there are patients at low risk of SM which may be relevant in clinical practice.
Methods
The European Competence Network on Mastocytosis (ECNM) registry is the largest collection of data on patients with mastocytosis. 1145 patients of at least 18 years with MIS who had a BMB within six months of diagnosis were included. Patients with advanced mastocytosis (ASM, MCL, SM-AHN) were excluded. We identified significant variables in univariate analysis and created a multivariate regression model using the whole population as a training and validation set (bootstrapping). A risk score was derived and the model and score were validated with receiver operating curve (ROC) area under the curve (AUC).
Results
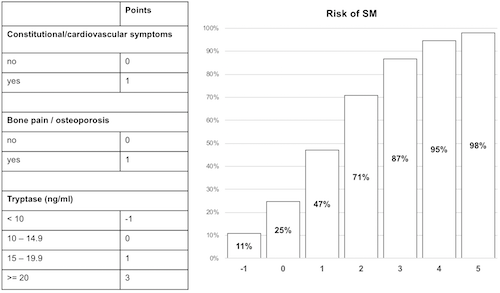
Of 1145 patients, 944 had SM and 201 had CM. Among these 1145 patients, 63.7% were female, with a median age of 44±13.3 years (18-81 years) and good performance status (ECOG 0-1, 97.6%). They were highly symptomatic (skin symptoms, 81.4%; other, 65.5%). Almost all patients had typical skin lesions (>97%) and tryptase levels varied greatly (median 29.3±81.9 ng/ml, range: 1-885). In a univariate analysis to determine predictors of SM in patients with MIS, significant variables were typical MIS (p=0.001), a positive Darier’s sign (p=0.020), constitutional/cardiovascular symptoms (p=0.002), bone symptoms/osteoporosis (p<0.001), gastrointestinal symptoms (p=0.004), serum tryptase (p<0.001), palpable spleen (p=0.003), age >65 years (p=0.009), lactate dehydrogenase (p=0.003), monocytes (p=0.001) and beta2-microglobulin (p=0.047). In the multivariate regression model, tryptase level (p<0.001), constitutional/cardiovascular symptoms (p=0.014) and bone symptoms/osteoporosis (p<0.001) were identified as independent predictors of SM (p<0.001, Nagelkerke R2=0.462, Hosmer-Lemeshow p=0.177). The model correctly classified 88.1% of the cases as SM (sensitivity 90.7%, specifity 69.1%, positive predictive value 95.5%, negative predictive value 50.3%). A risk score was derived and points were assigned to all variables, creating a six-point scale with a risk of SM ranging from 10.7% to 98.0% (Fig. 1). ROC AUC was 0.871 for the model and 0.867 for the risk score. Bootstrap validation (k=1000) led to an optimism-corrected AUC of 0.853 for the model and 0.849 for the score. Of all MIS patients, 17.4% (n=199) were at low risk (-1 and 0 points, 10.7 and 24.7% risk of SM), 14.5% (n=166) at medium risk (1 and 2 points, 47.1 and 70.7%) and 68.1% (n=779) at high risk (3-5 points, 86.8-98%).
Conclusion
Using a large data set of the ECNM registry, we created a risk score to better predict SM in patients with MIS. Although it will need validation in independent cohorts, our score seems to be valid and may discriminate between patients with SM and CM. Our new score may thus have clinical implications and may assist in the decision to recommend a BMB in adults with MIS.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Mast cell disease, Mastocytosis, Risk factor
Abstract: S813
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:30 - 12:45
Location: Room A2
Background
Mastocytosis is a clonal mast cell disease which, in adults, most commonly affects skin, bone marrow and other organs. Symptoms and serum tryptase levels vary, and there are patients with pure cutaneous mastocytosis (CM). It is generally appreciated that all adult patients with MIS require a bone marrow biopsy (BMB) in order to confirm the presence of SM. Although BMB is safe, it may be painful and cause complications. Existing risk scores are based on relatively few patients and a limited set of parameters.
Aims
We aimed at creating a simple and solid risk score to predict SM in adult patients with MIS. We hypothesized that there are patients at low risk of SM which may be relevant in clinical practice.
Methods
The European Competence Network on Mastocytosis (ECNM) registry is the largest collection of data on patients with mastocytosis. 1145 patients of at least 18 years with MIS who had a BMB within six months of diagnosis were included. Patients with advanced mastocytosis (ASM, MCL, SM-AHN) were excluded. We identified significant variables in univariate analysis and created a multivariate regression model using the whole population as a training and validation set (bootstrapping). A risk score was derived and the model and score were validated with receiver operating curve (ROC) area under the curve (AUC).
Results
Of 1145 patients, 944 had SM and 201 had CM. Among these 1145 patients, 63.7% were female, with a median age of 44±13.3 years (18-81 years) and good performance status (ECOG 0-1, 97.6%). They were highly symptomatic (skin symptoms, 81.4%; other, 65.5%). Almost all patients had typical skin lesions (>97%) and tryptase levels varied greatly (median 29.3±81.9 ng/ml, range: 1-885). In a univariate analysis to determine predictors of SM in patients with MIS, significant variables were typical MIS (p=0.001), a positive Darier’s sign (p=0.020), constitutional/cardiovascular symptoms (p=0.002), bone symptoms/osteoporosis (p<0.001), gastrointestinal symptoms (p=0.004), serum tryptase (p<0.001), palpable spleen (p=0.003), age >65 years (p=0.009), lactate dehydrogenase (p=0.003), monocytes (p=0.001) and beta2-microglobulin (p=0.047). In the multivariate regression model, tryptase level (p<0.001), constitutional/cardiovascular symptoms (p=0.014) and bone symptoms/osteoporosis (p<0.001) were identified as independent predictors of SM (p<0.001, Nagelkerke R2=0.462, Hosmer-Lemeshow p=0.177). The model correctly classified 88.1% of the cases as SM (sensitivity 90.7%, specifity 69.1%, positive predictive value 95.5%, negative predictive value 50.3%). A risk score was derived and points were assigned to all variables, creating a six-point scale with a risk of SM ranging from 10.7% to 98.0% (Fig. 1). ROC AUC was 0.871 for the model and 0.867 for the risk score. Bootstrap validation (k=1000) led to an optimism-corrected AUC of 0.853 for the model and 0.849 for the score. Of all MIS patients, 17.4% (n=199) were at low risk (-1 and 0 points, 10.7 and 24.7% risk of SM), 14.5% (n=166) at medium risk (1 and 2 points, 47.1 and 70.7%) and 68.1% (n=779) at high risk (3-5 points, 86.8-98%).

Conclusion
Using a large data set of the ECNM registry, we created a risk score to better predict SM in patients with MIS. Although it will need validation in independent cohorts, our score seems to be valid and may discriminate between patients with SM and CM. Our new score may thus have clinical implications and may assist in the decision to recommend a BMB in adults with MIS.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Mast cell disease, Mastocytosis, Risk factor


