
Contributions
Abstract: S809
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 11:30 - 11:45
Location: Room A2
Background
The British study of De-Escalation and Stopping Treatment with Imatinib, Nilotinib or sprYcel (DESTINY) recruited from December 2013 until April 2015. Entrants were aged 18 or over, in first chronic phase, on the same tyrosine kinase inhibitor (TKI) for at least 3 years since original diagnosis (except that 1 change was permitted if intolerant to the initial TKI), and with all BCR-ABL qPCR transcript levels (minimum of 3) < 0.1% in the 12 months before entry. If all these results were also <0.01%, they were assigned to the ‘MR4 group’ (as studied in many other studies); patients with one or more result between 0.1% and 0.01% were allocated to a ‘MMR but not MR4 group’ (not previously studied in a stopping trial). Entry criteria were thus virtually identical to the EUROSKI study except that patients with MMR but not MR4 were also separately eligible.
Aims
TKI treatment was de-escalated to 50% of the standard dose (imatinib 200mg daily, dasatinib 50mg daily or nilotinib 200mg twice daily) for 12 months, then stopped altogether for a further 24 months. Centralised PCR monitoring was carried out 1-2 monthly, expressed according to International Scale. Molecular recurrence was defined as the first of 2 consecutive samples >0.1%; this required recommencement of the relevant TKI at full dose.
Methods
174 patients (male 98; female 76) were recruited after giving informed consent from 20 UK centres. At entry, 148 patients were receiving imatinib, 16 nilotinib and 10 dasatinib, for a median duration of 6.8 years.
Results
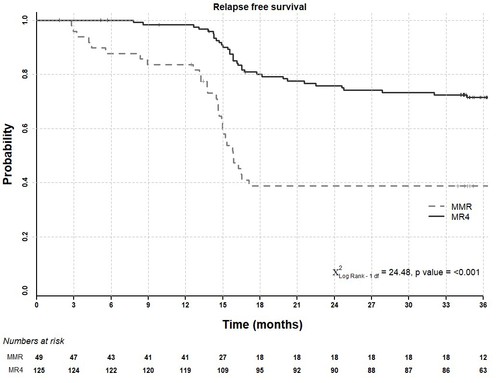
We previously reported that after 24 months on study, molecular recurrence was lower in patients with stable MR4 at entry (29 of 125 patients; 23.2%) than in those in MMR but not MR4 (29 of 49 patients; 59.2%) (p<0.001). We now show in the Figure below that during the subsequent 12 months of complete treatment cessation (i.e. months 25-36 of study), only 5 further recurrences have occurred, all in patients with stable MR4 at entry, giving a recurrence free survival (RFS) of 72% (90% CI: 65-79%) at 36 months follow-up in this group of patients. The overall recurrence rate is higher in the MMR but not MR4 group (20 of 36 patients during cessation; 39% RFS overall (90% CI: 29-52%); p = < 0.001), though no new recurrences have been seen in months 25-36 in this group.
Multivariable Cox proportional hazards modelling revealed that the baseline entry PCR result did not add to the predictive effect on RFS of the PCR pattern in the 12 months prior to trial entry; however the duration of TKI treatment was an additional predictive factor (p = 0.047; HR 0.93), as shown in other studies. The RFS probability remains unrelated to age, gender, performance status or prior TKI (imatinib vs second generation). No progression to advanced phase was seen; two deaths occurred due to unrelated causes, and one case lost haematological response. All relapsing patients have regained MMR within 4 months of resuming full dose TKI. No difference in RFS was seen between patients with a PCR level <0.0032% (MR4.5) at entry and those not.
Conclusion
The present finding of minimal (only 5) recurrences in the second stopping year, thus sustaining the excellent previously reported RFS out to 24 months of stopping, suggests that initial de-escalation is not simply delaying recurrence, though the mechanism of its benefit is not yet clear. Possibilities include gradual mobilisation of leukaemic stem cells into cycle and/or gradual improvement in the anti-leukaemic immune response at a time when TKI is still present. These require further study.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Treatment
Abstract: S809
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 11:30 - 11:45
Location: Room A2
Background
The British study of De-Escalation and Stopping Treatment with Imatinib, Nilotinib or sprYcel (DESTINY) recruited from December 2013 until April 2015. Entrants were aged 18 or over, in first chronic phase, on the same tyrosine kinase inhibitor (TKI) for at least 3 years since original diagnosis (except that 1 change was permitted if intolerant to the initial TKI), and with all BCR-ABL qPCR transcript levels (minimum of 3) < 0.1% in the 12 months before entry. If all these results were also <0.01%, they were assigned to the ‘MR4 group’ (as studied in many other studies); patients with one or more result between 0.1% and 0.01% were allocated to a ‘MMR but not MR4 group’ (not previously studied in a stopping trial). Entry criteria were thus virtually identical to the EUROSKI study except that patients with MMR but not MR4 were also separately eligible.
Aims
TKI treatment was de-escalated to 50% of the standard dose (imatinib 200mg daily, dasatinib 50mg daily or nilotinib 200mg twice daily) for 12 months, then stopped altogether for a further 24 months. Centralised PCR monitoring was carried out 1-2 monthly, expressed according to International Scale. Molecular recurrence was defined as the first of 2 consecutive samples >0.1%; this required recommencement of the relevant TKI at full dose.
Methods
174 patients (male 98; female 76) were recruited after giving informed consent from 20 UK centres. At entry, 148 patients were receiving imatinib, 16 nilotinib and 10 dasatinib, for a median duration of 6.8 years.
Results
We previously reported that after 24 months on study, molecular recurrence was lower in patients with stable MR4 at entry (29 of 125 patients; 23.2%) than in those in MMR but not MR4 (29 of 49 patients; 59.2%) (p<0.001). We now show in the Figure below that during the subsequent 12 months of complete treatment cessation (i.e. months 25-36 of study), only 5 further recurrences have occurred, all in patients with stable MR4 at entry, giving a recurrence free survival (RFS) of 72% (90% CI: 65-79%) at 36 months follow-up in this group of patients. The overall recurrence rate is higher in the MMR but not MR4 group (20 of 36 patients during cessation; 39% RFS overall (90% CI: 29-52%); p = < 0.001), though no new recurrences have been seen in months 25-36 in this group.
Multivariable Cox proportional hazards modelling revealed that the baseline entry PCR result did not add to the predictive effect on RFS of the PCR pattern in the 12 months prior to trial entry; however the duration of TKI treatment was an additional predictive factor (p = 0.047; HR 0.93), as shown in other studies. The RFS probability remains unrelated to age, gender, performance status or prior TKI (imatinib vs second generation). No progression to advanced phase was seen; two deaths occurred due to unrelated causes, and one case lost haematological response. All relapsing patients have regained MMR within 4 months of resuming full dose TKI. No difference in RFS was seen between patients with a PCR level <0.0032% (MR4.5) at entry and those not.

Conclusion
The present finding of minimal (only 5) recurrences in the second stopping year, thus sustaining the excellent previously reported RFS out to 24 months of stopping, suggests that initial de-escalation is not simply delaying recurrence, though the mechanism of its benefit is not yet clear. Possibilities include gradual mobilisation of leukaemic stem cells into cycle and/or gradual improvement in the anti-leukaemic immune response at a time when TKI is still present. These require further study.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Chronic myeloid leukemia, Treatment


