
Contributions
Abstract: S124
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:30 - 11:45
Location: Room A6
Background
High-dose melphalan (Mel) 200 mg/m2 is considered the standard of care preparative regimen for autologous hematopoietic stem cell transplantation (auto-HCT) for multiple myeloma. Two recent retrospective analyses suggested that a combination of busulfan (Bu) and Mel (Bu-Mel) may be associated with a longer progression-free survival (PFS) compared to Mel alone. In this randomized phase III trial we compared the safety and efficacy of Bu-Mel vs. Mel.
Aims
The primary objective was to compare PFS between the two arms.
Methods
Patients were randomized to either Bu-Mel or Mel using the Pocock-Simon method within 12 months of the start of induction therapy. The trial was designed to detect a 14-month increase in median PFS in the Bu-Mel arm with two-sided tests having nominal overall type I error .029 and power .80, with up to 3 tests using O’Brien-Fleming decision boundaries.
Results
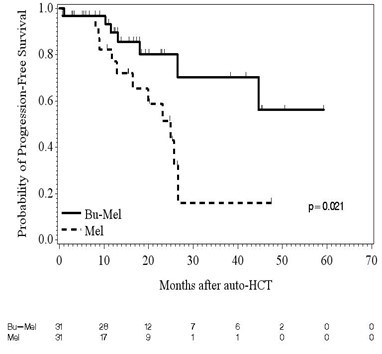
Two-hundred and four patients (Bu-Mel: 104, Mel: 100) were enrolled from October 2011 to March 2017. There was no significant difference between the two arms in age, gender, race, cytogenetic risk status, ISS stage, serum LDH, induction regimens, response to induction, or maintenance therapy. Sixty-two patients, 31 each in Bu-Mel (30%) and Mel (31%) arms, respectively, had high-risk (HR) chromosomal abnormalities as defined by IMWG criteria. At day 90 after auto-HCT, 11 (35%) HR MM patients had achieved complete remission (CR) in each arm (p=1.00). At last evaluation, 16 (52%) and 14 (45%) HR MM patients had achieved a CR (p=0.80) in Bu-Mel and Mel, respectively. Minimal residual disease (MRD) analysis by multiparametric flow cytometry (MFC) was performed at day 100 in 26 and 22 patients with HR MM in Bu-Mel and Mel, respectively. Fifteen (58%) and 13 (59%) achieved MRD negative status in Bu-Mel and Mel arms, respectively (p=1.00). -Meier estimates of median PFS for HR MM was not reached in the Bu-Mel arm, and 25.0 months in the Mel only arm (p=0.021) (Figure 1). This significant difference in PFS was maintained after adjusting for the type of maintenance therapy received (p=0.035). There was no difference in OS between HR MM patients in the Bu-Mel or Mel only arms (p=0.37). In a fitted Bayesian regression model that included age, race, ISS stage, and response to induction therapy, the posterior probability that patients receiving Bu-Mel had a smaller risk of progression and/or death compared with patients receiving Mel alone is 0.989, which is highly significant.
Conclusion
In this phase III trial, Bu-Mel regimen was safe, and associated with a significantly longer PFS than Mel alone. This significant difference in PFS was also observed for patients with high-risk disease. Potential explanations for a longer PFS without a significant difference in response rates include a deeper MRD negativity or selective targeting of clonogenic myeloma progenitor cells by Bu-Mel.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Autologous hematopoietic stem cell transplantation, Busulfan-melphalan, Multiple Myeloma
Abstract: S124
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:30 - 11:45
Location: Room A6
Background
High-dose melphalan (Mel) 200 mg/m2 is considered the standard of care preparative regimen for autologous hematopoietic stem cell transplantation (auto-HCT) for multiple myeloma. Two recent retrospective analyses suggested that a combination of busulfan (Bu) and Mel (Bu-Mel) may be associated with a longer progression-free survival (PFS) compared to Mel alone. In this randomized phase III trial we compared the safety and efficacy of Bu-Mel vs. Mel.
Aims
The primary objective was to compare PFS between the two arms.
Methods
Patients were randomized to either Bu-Mel or Mel using the Pocock-Simon method within 12 months of the start of induction therapy. The trial was designed to detect a 14-month increase in median PFS in the Bu-Mel arm with two-sided tests having nominal overall type I error .029 and power .80, with up to 3 tests using O’Brien-Fleming decision boundaries.
Results
Two-hundred and four patients (Bu-Mel: 104, Mel: 100) were enrolled from October 2011 to March 2017. There was no significant difference between the two arms in age, gender, race, cytogenetic risk status, ISS stage, serum LDH, induction regimens, response to induction, or maintenance therapy. Sixty-two patients, 31 each in Bu-Mel (30%) and Mel (31%) arms, respectively, had high-risk (HR) chromosomal abnormalities as defined by IMWG criteria. At day 90 after auto-HCT, 11 (35%) HR MM patients had achieved complete remission (CR) in each arm (p=1.00). At last evaluation, 16 (52%) and 14 (45%) HR MM patients had achieved a CR (p=0.80) in Bu-Mel and Mel, respectively. Minimal residual disease (MRD) analysis by multiparametric flow cytometry (MFC) was performed at day 100 in 26 and 22 patients with HR MM in Bu-Mel and Mel, respectively. Fifteen (58%) and 13 (59%) achieved MRD negative status in Bu-Mel and Mel arms, respectively (p=1.00). -Meier estimates of median PFS for HR MM was not reached in the Bu-Mel arm, and 25.0 months in the Mel only arm (p=0.021) (Figure 1). This significant difference in PFS was maintained after adjusting for the type of maintenance therapy received (p=0.035). There was no difference in OS between HR MM patients in the Bu-Mel or Mel only arms (p=0.37). In a fitted Bayesian regression model that included age, race, ISS stage, and response to induction therapy, the posterior probability that patients receiving Bu-Mel had a smaller risk of progression and/or death compared with patients receiving Mel alone is 0.989, which is highly significant.

Conclusion
In this phase III trial, Bu-Mel regimen was safe, and associated with a significantly longer PFS than Mel alone. This significant difference in PFS was also observed for patients with high-risk disease. Potential explanations for a longer PFS without a significant difference in response rates include a deeper MRD negativity or selective targeting of clonogenic myeloma progenitor cells by Bu-Mel.
Session topic: 23. Stem cell transplantation - Clinical
Keyword(s): Autologous hematopoietic stem cell transplantation, Busulfan-melphalan, Multiple Myeloma


