
Contributions
Abstract: S112
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Room A2
Background
Primary results of the randomized, phase 3, ECHELON-1 study demonstrated a significant improvement in modified progression-free survival (mPFS), per independent review facility (IRF), in patients with stage III and IV cHL treated with frontline A+AVD vs doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD). At 2 years, the mPFS rates were 82.1% (95% CI, 78.8 to 85.0) vs 77.2% (95% CI, 73.7 to 80.4), respectively, a 4.9% point difference (Hazard Ratio [HR] 0.77; 95% CI: 0.60–0.98; p=0.0351). We report data from pre-specified high risk subgroups of the ECHELON-1 data.
Aims
This prespecified subanalysis assessed the efficacy and safety of A+AVD vs ABVD in patients with cHL and high risk features including: ≥1 extranodal site of involvement, stage IV disease, or an International Prognostic Factor Project (IPFP) score of 4–7.
Methods
Patients were randomized 1:1 to receive up to six 28-day cycles of A+AVD (brentuximab vedotin 1.2 mg/kg, doxorubicin 25 mg/m2, vinblastine 6 mg/m2, dacarbazine 375 mg/m2) or ABVD (AVD regimen + bleomycin 10 units/m2) intravenously on Days 1 and 15 of each cycle. Patients were analyzed by Stage at diagnosis (III vs IV), IPFP score (0–1 vs 2–3 vs 4–7), and number of extranodal disease sites (0 vs 1 vs ≥1). The sub-group analyses were performed on the primary endpoint of mPFS (defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy).
Results
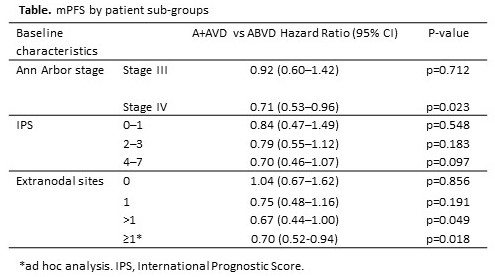
In total, 664 and 670 patients were randomized to A+AVD and ABVD, respectively. High risk features at baseline were well balanced between treatment group with 64% and 63% having stage IV disease, 25% and 27% having an International Prognostic Score (IPS) of 4–7 in the A+AVD and ABVD arms respectively, 62% in each arm had ≥1 extranodal sites at baseline. Most prespecified subgroups demonstrated a consistent trend toward benefit with A+AVD. A+AVD showed the most improved mPFS compared with ABVD in the following sub-groups (Table): stage IV disease (2-year mPFS 82.0% vs 75.3% [HR=0.71, 95% CI: 0.53–0.96; p=0.023]), >1 extranodal sites (2-year mPFS 80.2% vs 71.1% [HR=0.67, 95% CI: 0.44–1.00; p=0.049], ≥1 extranodal sites (2-year mPFS 82.4% vs 74.9% [HR=0.70, 95% CI: 0.52–0.94; p=0.02]). Patients with an IPS of 4–7 also had a favorable improvement in mPFS with A+AVD (77.0% vs 69.2% [HR=0.70, 95% CI: 0.46–1.07; p=0.097]). Efficacy and safety analyses for combinations of high risk sub-groups will be presented in full.
Conclusion
Compared with standard ABVD, A+AVD in frontline trends favorably for mPFS outcomes for patients with high risk cHL (Stage IV disease, or ≥1 extranodal disease sites, or IPS of 4–7) and the mPFS benefit for high risk patients is more than the ITT population. These results suggest that patients with high risk cHL might have a greater treatment benefit with A+AVD compared with ABVD.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): CD30, Clinical Trial, High risk, Hodgkin's Lymphoma
Abstract: S112
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Room A2
Background
Primary results of the randomized, phase 3, ECHELON-1 study demonstrated a significant improvement in modified progression-free survival (mPFS), per independent review facility (IRF), in patients with stage III and IV cHL treated with frontline A+AVD vs doxorubicin, bleomycin, vinblastine, dacarbazine (ABVD). At 2 years, the mPFS rates were 82.1% (95% CI, 78.8 to 85.0) vs 77.2% (95% CI, 73.7 to 80.4), respectively, a 4.9% point difference (Hazard Ratio [HR] 0.77; 95% CI: 0.60–0.98; p=0.0351). We report data from pre-specified high risk subgroups of the ECHELON-1 data.
Aims
This prespecified subanalysis assessed the efficacy and safety of A+AVD vs ABVD in patients with cHL and high risk features including: ≥1 extranodal site of involvement, stage IV disease, or an International Prognostic Factor Project (IPFP) score of 4–7.
Methods
Patients were randomized 1:1 to receive up to six 28-day cycles of A+AVD (brentuximab vedotin 1.2 mg/kg, doxorubicin 25 mg/m2, vinblastine 6 mg/m2, dacarbazine 375 mg/m2) or ABVD (AVD regimen + bleomycin 10 units/m2) intravenously on Days 1 and 15 of each cycle. Patients were analyzed by Stage at diagnosis (III vs IV), IPFP score (0–1 vs 2–3 vs 4–7), and number of extranodal disease sites (0 vs 1 vs ≥1). The sub-group analyses were performed on the primary endpoint of mPFS (defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy).
Results
In total, 664 and 670 patients were randomized to A+AVD and ABVD, respectively. High risk features at baseline were well balanced between treatment group with 64% and 63% having stage IV disease, 25% and 27% having an International Prognostic Score (IPS) of 4–7 in the A+AVD and ABVD arms respectively, 62% in each arm had ≥1 extranodal sites at baseline. Most prespecified subgroups demonstrated a consistent trend toward benefit with A+AVD. A+AVD showed the most improved mPFS compared with ABVD in the following sub-groups (Table): stage IV disease (2-year mPFS 82.0% vs 75.3% [HR=0.71, 95% CI: 0.53–0.96; p=0.023]), >1 extranodal sites (2-year mPFS 80.2% vs 71.1% [HR=0.67, 95% CI: 0.44–1.00; p=0.049], ≥1 extranodal sites (2-year mPFS 82.4% vs 74.9% [HR=0.70, 95% CI: 0.52–0.94; p=0.02]). Patients with an IPS of 4–7 also had a favorable improvement in mPFS with A+AVD (77.0% vs 69.2% [HR=0.70, 95% CI: 0.46–1.07; p=0.097]). Efficacy and safety analyses for combinations of high risk sub-groups will be presented in full.

Conclusion
Compared with standard ABVD, A+AVD in frontline trends favorably for mPFS outcomes for patients with high risk cHL (Stage IV disease, or ≥1 extranodal disease sites, or IPS of 4–7) and the mPFS benefit for high risk patients is more than the ITT population. These results suggest that patients with high risk cHL might have a greater treatment benefit with A+AVD compared with ABVD.
Session topic: 17. Hodgkin lymphoma – Clinical
Keyword(s): CD30, Clinical Trial, High risk, Hodgkin's Lymphoma


