
Contributions
Abstract: S134
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:30 - 11:45
Location: Room A8
Background
B Cell Maturation Antigen (BCMA) has appeared as a promising antigen to be used in CAR immunotherapy against Multiple Myeloma (MM) patients due to specific BCMA expression in plasma cells and its absence in most tissues. Recent clinical results infusing CART BCMA cells in patients with relapsed/refractory MM with a median of 7 prior lines of therapy (i.e.: NCT02658929) have shown 94% of objective responses, including 56% of complete remissions at 10 months. Deepening of response over time was detected. One of the main problems limiting the success of CART immunotherapy is the early disappearance of CART cells, occurring in approximately 30% of patients receiving CART19, which might be attenuated by the use of a humanized CART. Moreover, the high inflammatory response, termed cytokine release syndrome (CRS), could be detrimental to the patient and needs to be carefully monitored.
Aims
We decided to create a humanized CART-BCMA for the treatment of MM patients, and investigate possible ways of decreasing inflammation associated to CART expansion without impacting in the anti-MM CART activity.
Methods
We designed a 2nd generation murine CAR against BCMA with 4-1BB as co-stimulatory domain, and after confirming its functionality, we humanized the scFv of BCMA. In vitro studies were performed with MM cell lines (RPMI-8226, ARP1, U266) and K562 as a negative control cell line. In vivo studies were performed in NSG mice receiving 1·106 of MM (ARP1) cells i.v. injected at day 1. Then, mice received 5·106 i.v. injected of either CAR-T or Ctrl non-transduced T cells, both murine and humanized. CART cells were infused at day 6 and day 14 of MM injection to have an early and advanced MM model.
Results
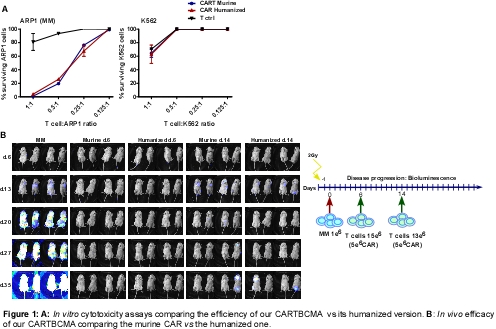
We successfully humanized the scFv of BCMA maintaining the cytotoxicity activity obtained by the murine CAR-BCMA. Moreover, both CARs were specific against MM (Fig. 1A), as K562 cells were not eliminated by CART cells. In vivo results showed that both murine and humanized CART-BCMA injected at day 6 were equally efficient in abrogating MM growth at an early-stage of the MM disease. When CART cells were infused at day 14, both murine and humanized CART-BCMA significantly reduced the advanced-stage of the MM disease but could not avoid the total progression of the disease (Fig. 1B). Moreover, a-MSH, a molecule with ant-inflammatory properties which does not decrease CD8 cytotoxic activity was evaluated as anti-inflammatory agent and its potential effect in CART activity. In vitro functional assays showed that a-MSH partially increased the cytotoxic activity of the CART-BCMA and decreased the IL6 and TNFa in vitro production.
Conclusion
We generated a humanized CART-BCMA that is as efficient as the murine one, but might favor a longer persistence of CART cells in patients. In the next months, this CAR will be translated into the clinic for MM patients. Furthermore, a-MSH potentially could be used as an adjuvant to ameliorate CRS without impacting negatively in the CART activity.
Session topic: 25. Gene therapy, cellular immunotherapy and vaccination – Biology & Translational Research
Keyword(s): Cancer immunotherapy, Lymphocyte, Mouse model, Multiple Myeloma
Abstract: S134
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 11:30 - 11:45
Location: Room A8
Background
B Cell Maturation Antigen (BCMA) has appeared as a promising antigen to be used in CAR immunotherapy against Multiple Myeloma (MM) patients due to specific BCMA expression in plasma cells and its absence in most tissues. Recent clinical results infusing CART BCMA cells in patients with relapsed/refractory MM with a median of 7 prior lines of therapy (i.e.: NCT02658929) have shown 94% of objective responses, including 56% of complete remissions at 10 months. Deepening of response over time was detected. One of the main problems limiting the success of CART immunotherapy is the early disappearance of CART cells, occurring in approximately 30% of patients receiving CART19, which might be attenuated by the use of a humanized CART. Moreover, the high inflammatory response, termed cytokine release syndrome (CRS), could be detrimental to the patient and needs to be carefully monitored.
Aims
We decided to create a humanized CART-BCMA for the treatment of MM patients, and investigate possible ways of decreasing inflammation associated to CART expansion without impacting in the anti-MM CART activity.
Methods
We designed a 2nd generation murine CAR against BCMA with 4-1BB as co-stimulatory domain, and after confirming its functionality, we humanized the scFv of BCMA. In vitro studies were performed with MM cell lines (RPMI-8226, ARP1, U266) and K562 as a negative control cell line. In vivo studies were performed in NSG mice receiving 1·106 of MM (ARP1) cells i.v. injected at day 1. Then, mice received 5·106 i.v. injected of either CAR-T or Ctrl non-transduced T cells, both murine and humanized. CART cells were infused at day 6 and day 14 of MM injection to have an early and advanced MM model.
Results
We successfully humanized the scFv of BCMA maintaining the cytotoxicity activity obtained by the murine CAR-BCMA. Moreover, both CARs were specific against MM (Fig. 1A), as K562 cells were not eliminated by CART cells. In vivo results showed that both murine and humanized CART-BCMA injected at day 6 were equally efficient in abrogating MM growth at an early-stage of the MM disease. When CART cells were infused at day 14, both murine and humanized CART-BCMA significantly reduced the advanced-stage of the MM disease but could not avoid the total progression of the disease (Fig. 1B). Moreover, a-MSH, a molecule with ant-inflammatory properties which does not decrease CD8 cytotoxic activity was evaluated as anti-inflammatory agent and its potential effect in CART activity. In vitro functional assays showed that a-MSH partially increased the cytotoxic activity of the CART-BCMA and decreased the IL6 and TNFa in vitro production.

Conclusion
We generated a humanized CART-BCMA that is as efficient as the murine one, but might favor a longer persistence of CART cells in patients. In the next months, this CAR will be translated into the clinic for MM patients. Furthermore, a-MSH potentially could be used as an adjuvant to ameliorate CRS without impacting negatively in the CART activity.
Session topic: 25. Gene therapy, cellular immunotherapy and vaccination – Biology & Translational Research
Keyword(s): Cancer immunotherapy, Lymphocyte, Mouse model, Multiple Myeloma


