
Contributions
Abstract: S131
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Room A7
Background
Hydroxyurea (HU) is considered first line cytoreductive therapy in most parts of the world for patients with Myeloproliferative Neoplasms (MPNs). The drug effectively lowers the risk of thrombosis but there is a concern with regard to its possible leukemogenic potential. Recombinant Interferon Alpha-2a (r-IFNα), which is used off label, is non-leukemogenic and has been demonstrated to induce high rates of both clinical, haematological and molecular responses in MPN patients.
Aims
To investigate efficacy and toxicity of low-dose r-IFNα compared to HU in patients with MPN in a randomized controlled clinical phase III trial.
Methods
Patients with newly diagnosed or previously untreated (cytoreductive agents) MPN according to WHO criteria were enrolled in the DALIAH trial (NCT01387763). Written informed consent was obtained from all patients. Patients > 60 years were randomized (I:I:I) to either r-IFNα-2a or r-IFNα-2b at a starting dose of 45 or 35 µg/week, respectively, or to HU at doses of 500 to 2000 mg/day. Patients ≤ 60 were randomized (I:I) to either r-IFNα-2a or r-IFNα-2b. The protocol allowed addition of HU in patients randomized to r-IFNα with major thrombosis or platelets > 1500 109/L. A planned interim analysis was performed after 18 months of therapy. The molecular and hematological response rates were assessed by the European Leukemia Net (ELN) 2009 and the EUMNET 2005 criteria. JAK2 V617F was analysed by qPCR.
Results
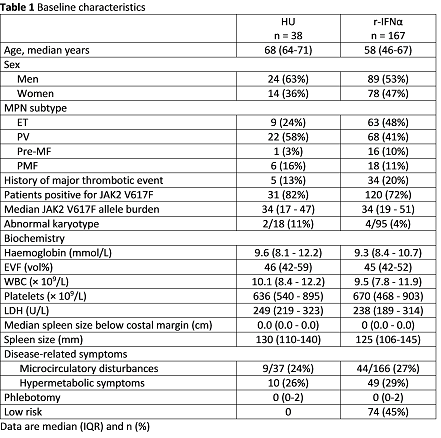
A total of 205 patients were enrolled between 2012 and 2015 (Table 1). The interim analysis was performed after a median of 17.7 months (range 17.1 – 18.2). Overall response rates (ORR) were 75% (24/32) for HU and 49% (73/149) for r-IFNα among patients with essential thrombocythemia (ET), polycythemia vera (PV) and prefibrotic myelofibrosis (pre-MF). Partial hematologic response (PHR) and complete hematologic response (CHR) were observed in 6 (19%) and 18 (56%) patients treated with HU and in 20 (13%) and 53 (36%) patients treated with r-IFNα. CHR was significantly higher in patients treated with HU (Fisher’s exact test, p=0.04). In primary myelofibrosis ORR was 100% (6/6) for patients on HU and 33% (6/18) for r-IFNα. For patients still on study medication at 18 months the ORR were 88% (30/34) for HU and 81% (79/98) for r-IFNα. The median treatment doses were 811 mg/day, (range 576 – 977) for HU and 53 µg/week (range 44 – 78) and 44 µg/week (range 33 – 46), for r-IFNα-2a or r-IFNα-2b, respectively. Ninety-six patients were available for molecular response analysis at 18 months. A complete molecular response was obtained in one patient treated with r-IFNα. Partial molecular response (PMR) was observed in 23% (7/31) and 16% (19/120) patients treated with HU and r-IFNα, respectively. The observed difference in PMR was not significant (Fisher’s exact test, p=0.42). Discontinuation of study medication for any reason after 18 months was 4 (11%) for HU and 69 (41%) for r-IFNα. Toxicity dependent drop-out was 5% for HU and 27% for r-IFNα. Grade 3-4 adverse events occurred in 7 (18%) HU patients and in 58 (35%) r-IFNα patients.
Conclusion
This planned analysis of efficacy and toxicity at 18 months shows a significant difference in the CHR rate (p=0.04) for patients treated with HU compared to low-dose r-IFNα. However, ORR was almost similar for patients still on study medication. Toxicity dependent discontinuation from r-IFNα was higher than expected (27%) even in this low-dose setting. Due to the study design patients treated with r-IFNα were younger than those treated with HU.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Hydroxyurea, Myeloproliferative disorder, Pegylated Interferon
Abstract: S131
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:00 - 12:15
Location: Room A7
Background
Hydroxyurea (HU) is considered first line cytoreductive therapy in most parts of the world for patients with Myeloproliferative Neoplasms (MPNs). The drug effectively lowers the risk of thrombosis but there is a concern with regard to its possible leukemogenic potential. Recombinant Interferon Alpha-2a (r-IFNα), which is used off label, is non-leukemogenic and has been demonstrated to induce high rates of both clinical, haematological and molecular responses in MPN patients.
Aims
To investigate efficacy and toxicity of low-dose r-IFNα compared to HU in patients with MPN in a randomized controlled clinical phase III trial.
Methods
Patients with newly diagnosed or previously untreated (cytoreductive agents) MPN according to WHO criteria were enrolled in the DALIAH trial (NCT01387763). Written informed consent was obtained from all patients. Patients > 60 years were randomized (I:I:I) to either r-IFNα-2a or r-IFNα-2b at a starting dose of 45 or 35 µg/week, respectively, or to HU at doses of 500 to 2000 mg/day. Patients ≤ 60 were randomized (I:I) to either r-IFNα-2a or r-IFNα-2b. The protocol allowed addition of HU in patients randomized to r-IFNα with major thrombosis or platelets > 1500 109/L. A planned interim analysis was performed after 18 months of therapy. The molecular and hematological response rates were assessed by the European Leukemia Net (ELN) 2009 and the EUMNET 2005 criteria. JAK2 V617F was analysed by qPCR.
Results
A total of 205 patients were enrolled between 2012 and 2015 (Table 1). The interim analysis was performed after a median of 17.7 months (range 17.1 – 18.2). Overall response rates (ORR) were 75% (24/32) for HU and 49% (73/149) for r-IFNα among patients with essential thrombocythemia (ET), polycythemia vera (PV) and prefibrotic myelofibrosis (pre-MF). Partial hematologic response (PHR) and complete hematologic response (CHR) were observed in 6 (19%) and 18 (56%) patients treated with HU and in 20 (13%) and 53 (36%) patients treated with r-IFNα. CHR was significantly higher in patients treated with HU (Fisher’s exact test, p=0.04). In primary myelofibrosis ORR was 100% (6/6) for patients on HU and 33% (6/18) for r-IFNα. For patients still on study medication at 18 months the ORR were 88% (30/34) for HU and 81% (79/98) for r-IFNα. The median treatment doses were 811 mg/day, (range 576 – 977) for HU and 53 µg/week (range 44 – 78) and 44 µg/week (range 33 – 46), for r-IFNα-2a or r-IFNα-2b, respectively. Ninety-six patients were available for molecular response analysis at 18 months. A complete molecular response was obtained in one patient treated with r-IFNα. Partial molecular response (PMR) was observed in 23% (7/31) and 16% (19/120) patients treated with HU and r-IFNα, respectively. The observed difference in PMR was not significant (Fisher’s exact test, p=0.42). Discontinuation of study medication for any reason after 18 months was 4 (11%) for HU and 69 (41%) for r-IFNα. Toxicity dependent drop-out was 5% for HU and 27% for r-IFNα. Grade 3-4 adverse events occurred in 7 (18%) HU patients and in 58 (35%) r-IFNα patients.

Conclusion
This planned analysis of efficacy and toxicity at 18 months shows a significant difference in the CHR rate (p=0.04) for patients treated with HU compared to low-dose r-IFNα. However, ORR was almost similar for patients still on study medication. Toxicity dependent discontinuation from r-IFNα was higher than expected (27%) even in this low-dose setting. Due to the study design patients treated with r-IFNα were younger than those treated with HU.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Hydroxyurea, Myeloproliferative disorder, Pegylated Interferon


