
Contributions
Abstract: S1564
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Victoria Hall
Background
Venetoclax (VEN) is a potent, small molecule BH3-mimetic targeting pro-survival BCL-2 with an emerging role for treatment of elderly patients with Acute Myeloid Leukemia (AML) unfit for intensive chemotherapy in combination with low-dose cytarabine (LDAC)(Wei et al, ASH 2017) or hypomethylating agents (HMA)(Di Nardo et al, Lancet Oncol 2018). The optimal dose of VEN was 600mg and 400mg in the LDAC and HMA studies, respectively. The feasibility of combining VEN with more intensified chemotherapy regimens has yet to be reported.
Aims
To evaluate the optimal dose, safety and efficacy of VEN in combination with dose-modified intensive chemotherapy in older patients with AML.
Methods
Treatment- Several factors were incorporated to mitigate the risk of hematologic toxicity and tumor lysis syndrome: 1) a 7-day VEN pre-phase incorporating dose ramp-up to achieve steady state prior to commencing chemotherapy; 2) dose escalation commenced at VEN 50mg (Cohort A), 100mg (B), 200mg (C), 400mg (D) and 600mg (E); 3) during induction, staggered and attenuated (Yoon et al, Am J Hematol 2013) addition of cytarabine (100mg/m2/d continuous IVI d8-12) and idarubicin (12mg/m2 IV d9-10); 4) a treatment-free VEN phase after d14 to allow hematopoietic recovery and 5) for patients in remission, further therapy with 4 cycles of “continuation” comprising 14 days of VEN at the cohort prescribed dose and bolus cytarabine 100mg/m2 IV (days 8-9) and idarubicin 12mg/m2 IV (day 8). After continuation, up to 7 cycles of VEN maintenance monotherapy were permitted. Antifungal azoles were prohibited during the period of VEN exposure in cohorts A-E. Eligibility- De novo or secondary AML (excluding APL), age ≥65 years (unless ≥60yo with monosomal karyotype), ECOG 0-1, adequate organ function, WCC <25 x 109/L, prior therapy for MDS/AML with HMA/LDAC was permitted after a 14-day washout. The first patient was enrolled on 17JUL2016. Dose-limiting toxicity (DLT)- Grade 4 neutropenia or thrombocytopenia lasting >42d after starting chemotherapy unrelated to residual AML/MDS and severe marrow hypocellularity <10%; other grade 4 toxicity related to VEN. Response- ELN 2010 response criteria.
Results
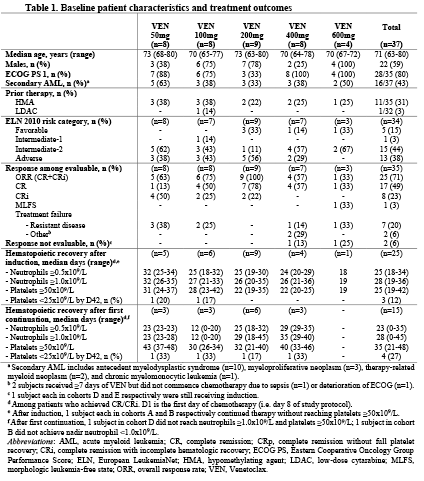
Data cut-off date was 13FEB2018. 37 patients were enrolled with 35 evaluable for response. Table 1 summarizes baseline characteristics of each cohort. Median age was 71 years (22% ≥75 years), 43% had secondary AML and 31% prior HMA failure. Grade ≥3 adverse events (≥10% of patients) during induction were thrombocytopenia (100%), neutropenia (100%), febrile neutropenia (70%), neutropenic sepsis (24%) and atrial fibrillation (14%). One hematologic DLT was reported in Cohort E (VEN 600 mg), with persistent neutropenia and thrombocytopenia beyond day 42 in a patient with transformed myelofibrosis. Maximum tolerated dose (MTD) has not yet been reached. Early mortality was 8% and 24% at 30 and 60 days, respectively. CR/CRi rate was 25/35 (71%) (Table 1). During the venetoclax pre-phase, the median relative bone marrow blast count change was -20% (range -70.5 to +14.5%). For those who achieved CR/CRi, the median time to both neutrophil (≥0.5x109/L) and platelet (≥50x109/L) recovery during induction was 25 days from day 1 of chemotherapy. Median follow-up of survivors was 9.2 months (range 0.3-17.9 months).
Conclusion
To date, the MTD has not been reached with VEN up to 600mg in combination with 5+2 chemotherapy in fit older patients with AML. The overall CR/CRi rate is 71%. Analyses for response duration and survival are ongoing.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AML, BCL2, Clinical Trial, Elderly
Abstract: S1564
Type: Oral Presentation
Presentation during EHA23: On Sunday, June 17, 2018 from 09:00 - 09:15
Location: Victoria Hall
Background
Venetoclax (VEN) is a potent, small molecule BH3-mimetic targeting pro-survival BCL-2 with an emerging role for treatment of elderly patients with Acute Myeloid Leukemia (AML) unfit for intensive chemotherapy in combination with low-dose cytarabine (LDAC)(Wei et al, ASH 2017) or hypomethylating agents (HMA)(Di Nardo et al, Lancet Oncol 2018). The optimal dose of VEN was 600mg and 400mg in the LDAC and HMA studies, respectively. The feasibility of combining VEN with more intensified chemotherapy regimens has yet to be reported.
Aims
To evaluate the optimal dose, safety and efficacy of VEN in combination with dose-modified intensive chemotherapy in older patients with AML.
Methods
Treatment- Several factors were incorporated to mitigate the risk of hematologic toxicity and tumor lysis syndrome: 1) a 7-day VEN pre-phase incorporating dose ramp-up to achieve steady state prior to commencing chemotherapy; 2) dose escalation commenced at VEN 50mg (Cohort A), 100mg (B), 200mg (C), 400mg (D) and 600mg (E); 3) during induction, staggered and attenuated (Yoon et al, Am J Hematol 2013) addition of cytarabine (100mg/m2/d continuous IVI d8-12) and idarubicin (12mg/m2 IV d9-10); 4) a treatment-free VEN phase after d14 to allow hematopoietic recovery and 5) for patients in remission, further therapy with 4 cycles of “continuation” comprising 14 days of VEN at the cohort prescribed dose and bolus cytarabine 100mg/m2 IV (days 8-9) and idarubicin 12mg/m2 IV (day 8). After continuation, up to 7 cycles of VEN maintenance monotherapy were permitted. Antifungal azoles were prohibited during the period of VEN exposure in cohorts A-E. Eligibility- De novo or secondary AML (excluding APL), age ≥65 years (unless ≥60yo with monosomal karyotype), ECOG 0-1, adequate organ function, WCC <25 x 109/L, prior therapy for MDS/AML with HMA/LDAC was permitted after a 14-day washout. The first patient was enrolled on 17JUL2016. Dose-limiting toxicity (DLT)- Grade 4 neutropenia or thrombocytopenia lasting >42d after starting chemotherapy unrelated to residual AML/MDS and severe marrow hypocellularity <10%; other grade 4 toxicity related to VEN. Response- ELN 2010 response criteria.
Results
Data cut-off date was 13FEB2018. 37 patients were enrolled with 35 evaluable for response. Table 1 summarizes baseline characteristics of each cohort. Median age was 71 years (22% ≥75 years), 43% had secondary AML and 31% prior HMA failure. Grade ≥3 adverse events (≥10% of patients) during induction were thrombocytopenia (100%), neutropenia (100%), febrile neutropenia (70%), neutropenic sepsis (24%) and atrial fibrillation (14%). One hematologic DLT was reported in Cohort E (VEN 600 mg), with persistent neutropenia and thrombocytopenia beyond day 42 in a patient with transformed myelofibrosis. Maximum tolerated dose (MTD) has not yet been reached. Early mortality was 8% and 24% at 30 and 60 days, respectively. CR/CRi rate was 25/35 (71%) (Table 1). During the venetoclax pre-phase, the median relative bone marrow blast count change was -20% (range -70.5 to +14.5%). For those who achieved CR/CRi, the median time to both neutrophil (≥0.5x109/L) and platelet (≥50x109/L) recovery during induction was 25 days from day 1 of chemotherapy. Median follow-up of survivors was 9.2 months (range 0.3-17.9 months).

Conclusion
To date, the MTD has not been reached with VEN up to 600mg in combination with 5+2 chemotherapy in fit older patients with AML. The overall CR/CRi rate is 71%. Analyses for response duration and survival are ongoing.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): AML, BCL2, Clinical Trial, Elderly


