
Contributions
Abstract: S880
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 17:00 - 17:15
Location: Room A7
Background
Although patients with chronic myelomonocytic leukemia (CMML) have surprisingly diverse genomic alterations, these events tend to occur in a limited number of pathways. The prognostic impact of pathway activation in CMML is poorly investigated. Recently we were able to show that the spontaneous in vitro growth of myeloid colonies (CFU-GM) may be a useful functional parameter of RAS pathway activation (Geissler K et al, Leukemia 2016).
Aims
To investigate the prognostic impact of RAS pathway activation in patients with CMML.
Methods
In this study we analyzed CMML patient samples which were collected in the “Austrian Biodatabase for CMML” (ABCMML) with regard to the presence of molecular aberrations in RASopathy genes by targeted next generation sequencing (NGS) and the presence of high spontaneous myeloid colony formation using semisolid in vitro cultures as described previously (Geissler K et al, J Exp Med 1996). From 225 CMML patients 288 samples (BMMNC or PBMNC) were analyzed by NGS and 207 samples (PBMNC) were used for in vitro cultures. Mutations with a VAF >5% were considered as positive in this study and autonomous CFU-GM formation >100/105 PBMNC was considered as high colony growth. Survival analysis was calculated from the sampling date. Molecular and functional data were correlated with patient survival using Cox regression analysis.
Results
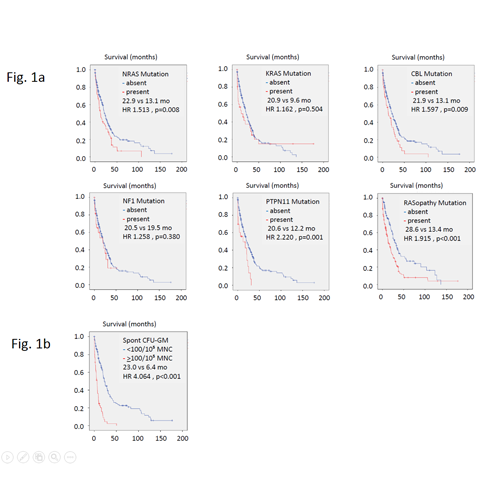
Fig 1a shows the Kaplan-Meier plots, hazard ratios and p-values of the prognostic impact of RASopathy gene mutations including NRAS (23.3%), KRAS (11.6%), CBL (16.5%), NF1 (10.2%), and PTPN11 (8.0%), respectively. If the presence of a mutation in at least one of the RASopathy genes was used as a composite molecular parameter (55.5%) the prognostic impact was most significant with a p value = 0.00006. The prognostic impact of RAS pathway activation could be also demonstrated at a functional level. As shown in Fig 1b high autonomous colony formation (CFU-GM >100/105 PBMNC; 27.0%) was significantly associated with inferior survival (p <0.00001).
Conclusion
Our data show that RAS pathway hyperactivation, which was demonstrated at the molecular and at the functional level, is associated with inferior survival in patients with CMML. These data may be important for better understanding the pathogenesis of CMML, for improving risk stratification of individual patients and for the design of targeted treatment concepts.
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): Chronic myelomonocytic leukemia, Colony assay, Ras
Abstract: S880
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 17:00 - 17:15
Location: Room A7
Background
Although patients with chronic myelomonocytic leukemia (CMML) have surprisingly diverse genomic alterations, these events tend to occur in a limited number of pathways. The prognostic impact of pathway activation in CMML is poorly investigated. Recently we were able to show that the spontaneous in vitro growth of myeloid colonies (CFU-GM) may be a useful functional parameter of RAS pathway activation (Geissler K et al, Leukemia 2016).
Aims
To investigate the prognostic impact of RAS pathway activation in patients with CMML.
Methods
In this study we analyzed CMML patient samples which were collected in the “Austrian Biodatabase for CMML” (ABCMML) with regard to the presence of molecular aberrations in RASopathy genes by targeted next generation sequencing (NGS) and the presence of high spontaneous myeloid colony formation using semisolid in vitro cultures as described previously (Geissler K et al, J Exp Med 1996). From 225 CMML patients 288 samples (BMMNC or PBMNC) were analyzed by NGS and 207 samples (PBMNC) were used for in vitro cultures. Mutations with a VAF >5% were considered as positive in this study and autonomous CFU-GM formation >100/105 PBMNC was considered as high colony growth. Survival analysis was calculated from the sampling date. Molecular and functional data were correlated with patient survival using Cox regression analysis.
Results
Fig 1a shows the Kaplan-Meier plots, hazard ratios and p-values of the prognostic impact of RASopathy gene mutations including NRAS (23.3%), KRAS (11.6%), CBL (16.5%), NF1 (10.2%), and PTPN11 (8.0%), respectively. If the presence of a mutation in at least one of the RASopathy genes was used as a composite molecular parameter (55.5%) the prognostic impact was most significant with a p value = 0.00006. The prognostic impact of RAS pathway activation could be also demonstrated at a functional level. As shown in Fig 1b high autonomous colony formation (CFU-GM >100/105 PBMNC; 27.0%) was significantly associated with inferior survival (p <0.00001).

Conclusion
Our data show that RAS pathway hyperactivation, which was demonstrated at the molecular and at the functional level, is associated with inferior survival in patients with CMML. These data may be important for better understanding the pathogenesis of CMML, for improving risk stratification of individual patients and for the design of targeted treatment concepts.
Session topic: 10. Myelodysplastic syndromes – Clinical
Keyword(s): Chronic myelomonocytic leukemia, Colony assay, Ras


