
Contributions
Abstract: S801
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:00 - 12:15
Location: Room A1
Background
In ZUMA-1 (NCT02348216), axi-cel, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, demonstrated significant benefit in patients with refractory large B cell lymphoma with an objective response rate (ORR) of 82% (complete response [CR] 58%; Neelapu & Locke et al. N Engl J Med. 2017). These results supported the recent approval of axi-cel by the US FDA for the treatment of adult patients with relapsed or refractory large B cell lymphoma after ≥ 2 prior lines of systemic therapy.
Aims
To assess outcomes of axi-cel by prior lines of therapy (LoT) in patients from Phases 1 and 2 of ZUMA-1.
Methods
All patients provided written informed consent. Patients with refractory large B cell lymphoma were leukapheresed and received 2 × 106 CAR T cells/kg after low-dose conditioning chemotherapy (Neelapu & Locke et al. N Engl J Med. 2017). Patients were evaluated by number of prior LoT: 2–3 vs ≥ 4. Autologous stem cell transplant (ASCT) was considered a prior LoT.
Results
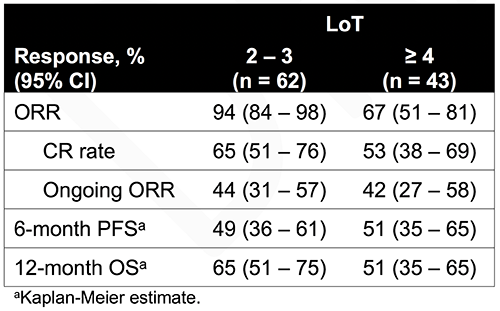
As of 8/11/17, median follow-up was 15.4 months for the 108 patients treated with axi-cel. Sixty-two (57%) patients had 2–3 prior LoT and 43 (40%) had ≥ 4. Patients with 2–3 and ≥ 4 prior LoT had median ages of 60 and 55 y, 65% and 47% of patients had ECOG performance status 1, 18% and 42% had prior ASCT, and 76% and 93% had disease stage III/IV, respectively. ORRs were 94% and 67% for patients with 2–3 and ≥ 4 prior LoT, respectively, with CR rates of 65% and 53%; 44% and 42% of patients had ongoing responses as of the data cutoff (Table). Overall survival (OS) at 12 months was 65% and 51% for patients with 2–3 and ≥ 4 prior LoT, respectively. Grade ≥ 3 treatment-emergent adverse events (AEs) were reported for nearly all (100% and 93%) patients with 2–3 and ≥ 4 LoT, with similar rates of Grade ≥ 3 cytokine release syndrome (11% and 12%) and neurologic events (32% and 30%). There were 1 and 3 Grade 5 AEs unrelated to disease progression in the 2–3 and ≥ 4 LoT groups, respectively.
Conclusion
Axi-cel demonstrated long-term clinical benefit for patients with refractory large B cell lymphoma, regardless of the number of prior LoT.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Cancer immunotherapy, CD19, Non-Hodgkin's lymphoma
Abstract: S801
Type: Oral Presentation
Presentation during EHA23: On Saturday, June 16, 2018 from 12:00 - 12:15
Location: Room A1
Background
In ZUMA-1 (NCT02348216), axi-cel, an anti-CD19 chimeric antigen receptor (CAR) T cell therapy, demonstrated significant benefit in patients with refractory large B cell lymphoma with an objective response rate (ORR) of 82% (complete response [CR] 58%; Neelapu & Locke et al. N Engl J Med. 2017). These results supported the recent approval of axi-cel by the US FDA for the treatment of adult patients with relapsed or refractory large B cell lymphoma after ≥ 2 prior lines of systemic therapy.
Aims
To assess outcomes of axi-cel by prior lines of therapy (LoT) in patients from Phases 1 and 2 of ZUMA-1.
Methods
All patients provided written informed consent. Patients with refractory large B cell lymphoma were leukapheresed and received 2 × 106 CAR T cells/kg after low-dose conditioning chemotherapy (Neelapu & Locke et al. N Engl J Med. 2017). Patients were evaluated by number of prior LoT: 2–3 vs ≥ 4. Autologous stem cell transplant (ASCT) was considered a prior LoT.
Results
As of 8/11/17, median follow-up was 15.4 months for the 108 patients treated with axi-cel. Sixty-two (57%) patients had 2–3 prior LoT and 43 (40%) had ≥ 4. Patients with 2–3 and ≥ 4 prior LoT had median ages of 60 and 55 y, 65% and 47% of patients had ECOG performance status 1, 18% and 42% had prior ASCT, and 76% and 93% had disease stage III/IV, respectively. ORRs were 94% and 67% for patients with 2–3 and ≥ 4 prior LoT, respectively, with CR rates of 65% and 53%; 44% and 42% of patients had ongoing responses as of the data cutoff (Table). Overall survival (OS) at 12 months was 65% and 51% for patients with 2–3 and ≥ 4 prior LoT, respectively. Grade ≥ 3 treatment-emergent adverse events (AEs) were reported for nearly all (100% and 93%) patients with 2–3 and ≥ 4 LoT, with similar rates of Grade ≥ 3 cytokine release syndrome (11% and 12%) and neurologic events (32% and 30%). There were 1 and 3 Grade 5 AEs unrelated to disease progression in the 2–3 and ≥ 4 LoT groups, respectively.

Conclusion
Axi-cel demonstrated long-term clinical benefit for patients with refractory large B cell lymphoma, regardless of the number of prior LoT.
Session topic: 21. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Cancer immunotherapy, CD19, Non-Hodgkin's lymphoma


