
Contributions
Abstract: S104
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:30 - 12:45
Location: Room A1
Background
There is an unmet need for more effective therapeutic options for patients (pts) with non-Hodgkin lymphoma (NHL), including pts with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and indolent lymphomas (Crump et al, Blood 2017). Pts with follicular lymphoma (FL) experiencing early relapse (ER) within 2 years of initial diagnosis and those double refractory (DR) to both rituximab and chemotherapy, have limited treatment options and particularly poor outcomes (Casulo et al, JCO 2015). CC-122 is a novel oral agent that modulates cereblon, resulting in the selective ubiquitination and degradation of the hematopoietic transcription factors, Aiolos and Ikaros (Hagner et al, Blood 2015; Ribrag et al, Blood 2014). Preliminary results from the CC-122-NHL-001 trial, the first study of CC-122 in combination with obinutuzumab, showed promising response rates in pts with R/R B-cell NHL (Michot et al, Blood 2017).
Aims
To report updated safety and efficacy from the CC-122-NHL-001 phase Ib study of CC-122 plus obinutuzumab in pts with R/R B-cell NHL (EUDRACT 2014-003333-26; NCT02417285).
Methods
Eligible pts had histologically/cytologically confirmed CD20+ B-cell R/R NHL after ≥1 prior regimen for FL/marginal zone lymphoma (MZL) or after ≥2 regimens and/or ASCT for DLBCL. Pts received dose escalated CC-122 active ingredient in capsule formulation (AIC) 1, 2, 3, or 4 mg and formulated capsule (F6) 3 or 4 mg for 5 out of 7 days (5/7d) per week in 28-day cycles with a fixed dose of obinutuzumab IV 1000 mg on days 2, 8, 15 of cycle 1 and day 1 of cycles 2-8. Primary endpoints included safety, NTD, and MTD. Response was assessed by Cheson 2007 criteria.
Results
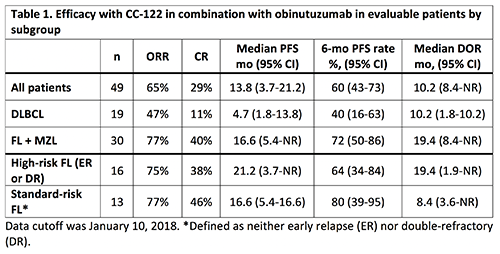
As of January 10, 2018, 49 R/R B-cell NHL pts were enrolled, including 29 pts with FL (59%), 19 (39%) with DLBCL, and 1 with MZL. Of the 29 FL pts, 16 were characterized as ER or DR and considered high-risk pts. For all pts, the median age was 60 y (range, 26–83), 32 (65%) were male, and 38 (78%) had stage III/IV disease. The median number of prior anticancer therapies was 3 (range, 1–11), and 19 pts (39%) had 1 prior ASCT. As of data cutoff, 16 pts (33%) were ongoing. The median number of cycles of CC-122 was 6 (range, 1-29). Although 16 pts (33%) had ≥1 dose reduction (31% due to AEs), these pts remained on treatment and experienced clinical benefit. Forty-one pts (84%) had dose interruptions (71% due to AEs). Two pts had a DLT, consisting of grade 4 neutropenia (n=1, CC-122 AIC 3 mg) and grade 5 tumor flare (n=1, CC-122 F6 4 mg). The most common any-grade AEs included neutropenia (65%), thrombocytopenia (39%), and diarrhea (29%). Grade 3/4 TEAEs occurred in 41 (84%) pts; the most common (≥15%) were neutropenia (55%) and thrombocytopenia (22%). 47% of pts had SAEs. The ORR was 65% with a CR rate of 29%; median PFS was 13.8 mo (95% CI, 3.7-21.2) and the median DOR was 10.2 mo (95% CI, 8.4-NR) (Table 1). Among pts with FL, the ORR was 77% with a CR rate of 40%. A subgroup analysis of efficacy was performed to further examine the activity of the combination in standard-risk (no ER/DR) and high-risk (ER or DR) pts with FL, demonstrating similar outcomes in the FL subgroups (Table 1).
Conclusion
The chemotherapy-free combination of CC-122 and obinutuzumab was well-tolerated and had favorable clinical activity and durable remissions in R/R B-cell NHL. Notably, subgroup analysis demonstrated that high-risk and standard-risk FL pts have comparable efficacy in response to CC-122 plus obinutuzumab. An expansion (Part B) study is ongoing for pts with R/R FL.
Session topic: 20. Indolent Non-Hodgkin lymphoma – Clinical
Keyword(s): B cell lymphoma, Non-Hodgkin's lymphoma, Obinutuzumab, Relapse
Abstract: S104
Type: Oral Presentation
Presentation during EHA23: On Friday, June 15, 2018 from 12:30 - 12:45
Location: Room A1
Background
There is an unmet need for more effective therapeutic options for patients (pts) with non-Hodgkin lymphoma (NHL), including pts with relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) and indolent lymphomas (Crump et al, Blood 2017). Pts with follicular lymphoma (FL) experiencing early relapse (ER) within 2 years of initial diagnosis and those double refractory (DR) to both rituximab and chemotherapy, have limited treatment options and particularly poor outcomes (Casulo et al, JCO 2015). CC-122 is a novel oral agent that modulates cereblon, resulting in the selective ubiquitination and degradation of the hematopoietic transcription factors, Aiolos and Ikaros (Hagner et al, Blood 2015; Ribrag et al, Blood 2014). Preliminary results from the CC-122-NHL-001 trial, the first study of CC-122 in combination with obinutuzumab, showed promising response rates in pts with R/R B-cell NHL (Michot et al, Blood 2017).
Aims
To report updated safety and efficacy from the CC-122-NHL-001 phase Ib study of CC-122 plus obinutuzumab in pts with R/R B-cell NHL (EUDRACT 2014-003333-26; NCT02417285).
Methods
Eligible pts had histologically/cytologically confirmed CD20+ B-cell R/R NHL after ≥1 prior regimen for FL/marginal zone lymphoma (MZL) or after ≥2 regimens and/or ASCT for DLBCL. Pts received dose escalated CC-122 active ingredient in capsule formulation (AIC) 1, 2, 3, or 4 mg and formulated capsule (F6) 3 or 4 mg for 5 out of 7 days (5/7d) per week in 28-day cycles with a fixed dose of obinutuzumab IV 1000 mg on days 2, 8, 15 of cycle 1 and day 1 of cycles 2-8. Primary endpoints included safety, NTD, and MTD. Response was assessed by Cheson 2007 criteria.
Results
As of January 10, 2018, 49 R/R B-cell NHL pts were enrolled, including 29 pts with FL (59%), 19 (39%) with DLBCL, and 1 with MZL. Of the 29 FL pts, 16 were characterized as ER or DR and considered high-risk pts. For all pts, the median age was 60 y (range, 26–83), 32 (65%) were male, and 38 (78%) had stage III/IV disease. The median number of prior anticancer therapies was 3 (range, 1–11), and 19 pts (39%) had 1 prior ASCT. As of data cutoff, 16 pts (33%) were ongoing. The median number of cycles of CC-122 was 6 (range, 1-29). Although 16 pts (33%) had ≥1 dose reduction (31% due to AEs), these pts remained on treatment and experienced clinical benefit. Forty-one pts (84%) had dose interruptions (71% due to AEs). Two pts had a DLT, consisting of grade 4 neutropenia (n=1, CC-122 AIC 3 mg) and grade 5 tumor flare (n=1, CC-122 F6 4 mg). The most common any-grade AEs included neutropenia (65%), thrombocytopenia (39%), and diarrhea (29%). Grade 3/4 TEAEs occurred in 41 (84%) pts; the most common (≥15%) were neutropenia (55%) and thrombocytopenia (22%). 47% of pts had SAEs. The ORR was 65% with a CR rate of 29%; median PFS was 13.8 mo (95% CI, 3.7-21.2) and the median DOR was 10.2 mo (95% CI, 8.4-NR) (Table 1). Among pts with FL, the ORR was 77% with a CR rate of 40%. A subgroup analysis of efficacy was performed to further examine the activity of the combination in standard-risk (no ER/DR) and high-risk (ER or DR) pts with FL, demonstrating similar outcomes in the FL subgroups (Table 1).

Conclusion
The chemotherapy-free combination of CC-122 and obinutuzumab was well-tolerated and had favorable clinical activity and durable remissions in R/R B-cell NHL. Notably, subgroup analysis demonstrated that high-risk and standard-risk FL pts have comparable efficacy in response to CC-122 plus obinutuzumab. An expansion (Part B) study is ongoing for pts with R/R FL.
Session topic: 20. Indolent Non-Hodgkin lymphoma – Clinical
Keyword(s): B cell lymphoma, Non-Hodgkin's lymphoma, Obinutuzumab, Relapse


