TRACEABILITY OF RED BLOOD CELLS IN A HOSPITAL TRANSFUSION LABORATORY (HTL)
(Abstract release date: 05/18/17)
EHA Library. Argyrou A. 05/18/17; 182944; PB2231

Dr. Aspasia Argyrou
Contributions
Contributions
Abstract
Abstract: PB2231
Type: Publication Only
Background
According to European legislation (2002/98/EC, 2005/61/EC) as a requirement of hemovigilance system traceability (confirmation of final destination of blood components in hospitals) information should be kept for 30 years, improving the quality and safety of the transfusion process.
Various methods are available from simple paper–based procedures to full electronic blood tracking systems.
The ideal goal is to trace the final fate of 100% of the red blood cell (RBC) units, from donor to recipient and vice versa.
Aims
To check the ability to trace each individual unit from donor to recipient or disposal in our hospital.
Methods
To ensure compliance, the minimum traceability data set for retention is a mix of
1) Wards’ paper files (file of transfusions and/or patient records: 14/2 wards respectively)
2) HTL electronic records and paper records
The transfusion practitioner is responsible for the collection and maintenance of traceability data.
Results
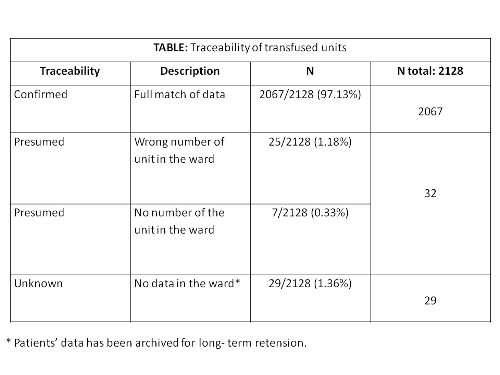
During the year 2016, the number of RBC units transfused in our hospital was 2128. The traceability status of the transfused units is shown in the table.
Conclusion
Although we are satisfied that the results represent a reasonably accurate working model of the current situation, the trail of a unit is less reliable after blood has left the HTL.
1. Patients’ notes to provide traceability are not totally reliable. It is apparent that the ward staff plays a key- role part in the chain and this highlights the need for them to receive training to emphasize the importance of their contribution to hospital compliance.
2. The indications are that the essential requirements on traceability are not fully met by the current laboratory computer system. A configuration is needed to produce a report which lists components which have been assigned for use but do not have an entry for return to stock or final fate. Ongoing problems will be referred to the Hospital Transfusion Committee.
3. For the longer term ultimately only effective IT system in both wards and HTL can ensure total traceability and we recommend the inclusion of electronic tracking system in the National Blood Donor Registry Programme (EMA)
Session topic: 30. Transfusion medicine
Keyword(s): Safety, Red blood cell, Blood transfusion
Abstract: PB2231
Type: Publication Only
Background
According to European legislation (2002/98/EC, 2005/61/EC) as a requirement of hemovigilance system traceability (confirmation of final destination of blood components in hospitals) information should be kept for 30 years, improving the quality and safety of the transfusion process.
Various methods are available from simple paper–based procedures to full electronic blood tracking systems.
The ideal goal is to trace the final fate of 100% of the red blood cell (RBC) units, from donor to recipient and vice versa.
Aims
To check the ability to trace each individual unit from donor to recipient or disposal in our hospital.
Methods
To ensure compliance, the minimum traceability data set for retention is a mix of
1) Wards’ paper files (file of transfusions and/or patient records: 14/2 wards respectively)
2) HTL electronic records and paper records
The transfusion practitioner is responsible for the collection and maintenance of traceability data.
Results
During the year 2016, the number of RBC units transfused in our hospital was 2128. The traceability status of the transfused units is shown in the table.

Conclusion
Although we are satisfied that the results represent a reasonably accurate working model of the current situation, the trail of a unit is less reliable after blood has left the HTL.
1. Patients’ notes to provide traceability are not totally reliable. It is apparent that the ward staff plays a key- role part in the chain and this highlights the need for them to receive training to emphasize the importance of their contribution to hospital compliance.
2. The indications are that the essential requirements on traceability are not fully met by the current laboratory computer system. A configuration is needed to produce a report which lists components which have been assigned for use but do not have an entry for return to stock or final fate. Ongoing problems will be referred to the Hospital Transfusion Committee.
3. For the longer term ultimately only effective IT system in both wards and HTL can ensure total traceability and we recommend the inclusion of electronic tracking system in the National Blood Donor Registry Programme (EMA)
Session topic: 30. Transfusion medicine
Keyword(s): Safety, Red blood cell, Blood transfusion
{{ help_message }}
{{filter}}


