ALLOGENEIC HEMATOPOIETIC STEM CELL TRANSPLANTATION FOR SECONDARY HAEMATOLOGICAL NEOPLASIA (T-HN): A SINGLE CENTER EXPERIENCE
(Abstract release date: 05/18/17)
EHA Library. Sica S. 05/18/17; 182875; PB2162

Simona Sica
Contributions
Contributions
Abstract
Abstract: PB2162
Type: Publication Only
Background
Therapy related haematological neoplasms (t-HN) occur due to direct mutational events of chemotherapeutic agents and radiotherapy. Disease latency, mutational events and prognosis vary with drugs categories.
Aims
The aim of this retrospective study was to assess the outcome of t-HN after hematopoietic stem cell transplantation (HSCT).
Methods
We describe a cohort of 31 patients, 19 females (61.3%) and 12 males (38.7%), with median age of 53 years (range, 20 to 64), who received an allogeneic HSCT in our Unit, between September 1999 and July 2016. Patients had a history of solid tumor in 15 cases (48.4%), haematological malignancies in 15 cases (48.4%) and both of them in one case (3.2%). All but one received a median of 2 (range, 1 to 6) lines of therapy. After a median of 36 months (range 12-190) from the first neoplasia, patients developed t-AML (n=19) (61.3%), t-Ph+ ALL (n=1) (3.2%), or t-MDS (n=11) (35.5%). Molecular abnormalities were detected in 7 (46.7%) out of 15 evaluable patients: BCR/ABL (1), ITD FLT3 (2), inv16 (1), NPM1 (2), NPM1+/ITD FLT3 (1). Karyotype aberrations were found in 18 (64.3%) out of 28 evaluable patients: 16.7% was favourable risk (n=3), 27.8% was intermediate risk (n=5) and 55.5% was adverse risk (n=10). The disease status at transplant was as follows: complete remission (n=13) (42%), refractory disease (N=10) (32%), stable disease (n=3) (10%). Patients received conventional chemotherapy in 14 cases (45.2%), azacytidine in 11 cases (35.5%), both of them in one case (3.2%), whereas 5 patients (16.1%) were untreated. The conditioning was myeloablative (MAC) in 20 patients (64.5%) or reduced intensity (RIC) in 11 patients (35.5%); the donor was a family member (REL) in 17 patients (54.8%) or unrelated (MUD) in 14 patients (45.2%). The hematopoietic cell transplantation comorbidity index (HCT-CI) was as follows: 14 patients (45.2%) had a score of 3 and 17 patients (54.8%) had a score of 4 or more. Overall survival was calculated with Kaplan-Meier method. Transplant-related mortality (TRM) and relapse-related mortality (RRD) rates were estimated by cumulative incidence (CI), both considering the opposite event as competing. Fine and Gray’s method for CI of TRM and RRD was used to evaluate the risk factors on univariate analysis.
Results
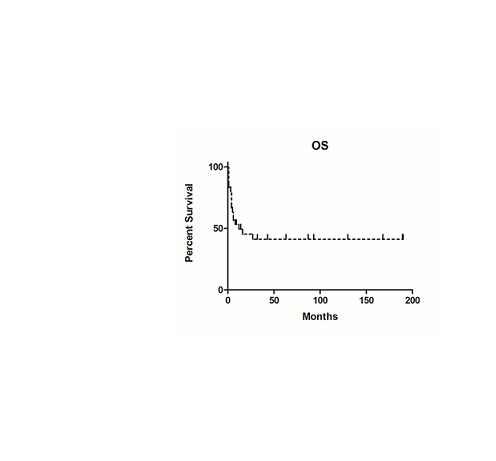
Twenty-three patients were in remission on day +30, by bone marrow cytology, 3 patients were classified as resistant disease and five patients were not evaluable because of early death. Five patients (21.7%) relapsed after a median 6 months (range, 3 to 15). At the time of this analysis (December 2016) 14 patients were alive with a median OS of 53 months (range 8-190), while 17 patients died after a median of 4 months (range 1-27): RRD was 16% (n=5) and TRM was 39% (n=12). Non relapse causes of death were as follows: GvHD (n=3), infectious complications (n=8) and EBV-related PTLD (n=1). One patient experienced a third tumor (breast cancer) thirteen years from HSCT. TRM was higher for patients transplanted from MUD (66%) as compared to REL donor (16%) (p=0.01). The overall survival was 45.2% (Figure 1) and 58% maintained a complete remission.
Conclusion
This report confirms that allogeneic HSCT is a curative approach in approximately 50% of patients with therapy related haematological neoplasms, especially for those patients who benefit from a familial donor.
Session topic: 22. Stem cell transplantation - Clinical
Keyword(s): Therapy-related AML, Second malignancy, Remission, Allogeneic hematopoietic stem cell transplant
Abstract: PB2162
Type: Publication Only
Background
Therapy related haematological neoplasms (t-HN) occur due to direct mutational events of chemotherapeutic agents and radiotherapy. Disease latency, mutational events and prognosis vary with drugs categories.
Aims
The aim of this retrospective study was to assess the outcome of t-HN after hematopoietic stem cell transplantation (HSCT).
Methods
We describe a cohort of 31 patients, 19 females (61.3%) and 12 males (38.7%), with median age of 53 years (range, 20 to 64), who received an allogeneic HSCT in our Unit, between September 1999 and July 2016. Patients had a history of solid tumor in 15 cases (48.4%), haematological malignancies in 15 cases (48.4%) and both of them in one case (3.2%). All but one received a median of 2 (range, 1 to 6) lines of therapy. After a median of 36 months (range 12-190) from the first neoplasia, patients developed t-AML (n=19) (61.3%), t-Ph+ ALL (n=1) (3.2%), or t-MDS (n=11) (35.5%). Molecular abnormalities were detected in 7 (46.7%) out of 15 evaluable patients: BCR/ABL (1), ITD FLT3 (2), inv16 (1), NPM1 (2), NPM1+/ITD FLT3 (1). Karyotype aberrations were found in 18 (64.3%) out of 28 evaluable patients: 16.7% was favourable risk (n=3), 27.8% was intermediate risk (n=5) and 55.5% was adverse risk (n=10). The disease status at transplant was as follows: complete remission (n=13) (42%), refractory disease (N=10) (32%), stable disease (n=3) (10%). Patients received conventional chemotherapy in 14 cases (45.2%), azacytidine in 11 cases (35.5%), both of them in one case (3.2%), whereas 5 patients (16.1%) were untreated. The conditioning was myeloablative (MAC) in 20 patients (64.5%) or reduced intensity (RIC) in 11 patients (35.5%); the donor was a family member (REL) in 17 patients (54.8%) or unrelated (MUD) in 14 patients (45.2%). The hematopoietic cell transplantation comorbidity index (HCT-CI) was as follows: 14 patients (45.2%) had a score of 3 and 17 patients (54.8%) had a score of 4 or more. Overall survival was calculated with Kaplan-Meier method. Transplant-related mortality (TRM) and relapse-related mortality (RRD) rates were estimated by cumulative incidence (CI), both considering the opposite event as competing. Fine and Gray’s method for CI of TRM and RRD was used to evaluate the risk factors on univariate analysis.
Results
Twenty-three patients were in remission on day +30, by bone marrow cytology, 3 patients were classified as resistant disease and five patients were not evaluable because of early death. Five patients (21.7%) relapsed after a median 6 months (range, 3 to 15). At the time of this analysis (December 2016) 14 patients were alive with a median OS of 53 months (range 8-190), while 17 patients died after a median of 4 months (range 1-27): RRD was 16% (n=5) and TRM was 39% (n=12). Non relapse causes of death were as follows: GvHD (n=3), infectious complications (n=8) and EBV-related PTLD (n=1). One patient experienced a third tumor (breast cancer) thirteen years from HSCT. TRM was higher for patients transplanted from MUD (66%) as compared to REL donor (16%) (p=0.01). The overall survival was 45.2% (Figure 1) and 58% maintained a complete remission.

Conclusion
This report confirms that allogeneic HSCT is a curative approach in approximately 50% of patients with therapy related haematological neoplasms, especially for those patients who benefit from a familial donor.
Session topic: 22. Stem cell transplantation - Clinical
Keyword(s): Therapy-related AML, Second malignancy, Remission, Allogeneic hematopoietic stem cell transplant
{{ help_message }}
{{filter}}


