
Contributions
Abstract: PB2146
Type: Publication Only
Background
Sickle Cell Disease (SCD) is an inherited blood disorder affecting millions of people. Sevuparin/DF02 is being developed to treat people suffering from SCD and is currently in clinical phase 2 for the treatment of the acute painful crisis in hospitalized SCD patients with intravenous infusion. This is called the Resolve program. In a second program called EASE, sevuparin/DF02 will be investigated as an on-demand treatment of early symptoms of painful sickle cell crisis in an at-home setting via a subcutaneous injection.Searching in the literature and discussing with health care providers, it becomes clear that little is known about how the SCD patients sense these early symptoms of a painful crisis. In order to gain increased understanding of how people living with SCD experience daily life, coping with disease, support by health care providers and the demand for new therapies, a patient survey addressing these areas was conducted.
Aims
The aim with this survey was to gain deeper understanding of different aspects of life with SCD by providing a channel for patients to air their own views. The outcome will provide important information and, in combination with future feasibility studies, will guide the design of the first clinical study aimed at treating the early symptoms of pain crises in SCD patients.
Methods
A 29-question survey was created to gather input on a wide variety of topics related to the lives of people living with SCD. This questionnaire was developed by Modus Therapeutics AB, Sweden, in conjunction with Micromattie Consulting Inc., USA. Experts and leaders of community-based organizations participated in two focus group sessions to ensure that the text and structure were ethical and appropriate for the intended purpose. The survey was hosted at www.modustxpatientsurvey.com. Patients answered the survey directly, or had their views entered in by a caregiver. The answers are anonymous. During the initial period, survey promotion occurred within the Sickle Cell Warriors online community and later, additional connections within the network of community-based organizations were leveraged. The survey was open for access during the period of January 10, 2017 through March 1, 2017.
Results
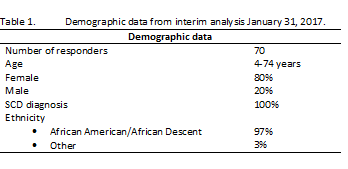
An interim analysis was conducted on January 31, 2017. Basic demographic data is presented in table 1. Responders were located mainly in the US. Medical history related questions indicate that fatigue (40%), aches/pain (37%), irritability (27%) and appetite (20%) are early symptoms and increase just before the onset of a pain crises. However, 7% reported infrequent signs and 19% never experienced an indicator of pain crisis. Patients take initiative at home to manage the onset of an acute crisis and the top 5 home strategies reported were: prescription pain medication (15%), sleep/rest (15%), apply heat using heating pad/blanket/bath/shower (13%), increase fluid intake (12%), and finally avoid stress (9%). Further it is clear, that people living with SCD are motivated to try a new therapy that could provide “significant relief” and “prevent symptoms from happening” due to their SCD.
Conclusion
The survey collected feedback about topics for which the patient is the best source of information. It is obvious that people with SCD are willing to self-medicate by subcutaneous injections and that there is a need for new tools and medications. With support from the answers from the survey, specific aspects will be considered while designing a first clinical study for subcutaneous sevuparin/DF02 administration to treat early symptoms of painful crisis in an at-home setting.
Session topic: 25. Sickle cell disease
Keyword(s): sickle cell disease, Patient, Development, Clinical Trial
Abstract: PB2146
Type: Publication Only
Background
Sickle Cell Disease (SCD) is an inherited blood disorder affecting millions of people. Sevuparin/DF02 is being developed to treat people suffering from SCD and is currently in clinical phase 2 for the treatment of the acute painful crisis in hospitalized SCD patients with intravenous infusion. This is called the Resolve program. In a second program called EASE, sevuparin/DF02 will be investigated as an on-demand treatment of early symptoms of painful sickle cell crisis in an at-home setting via a subcutaneous injection.Searching in the literature and discussing with health care providers, it becomes clear that little is known about how the SCD patients sense these early symptoms of a painful crisis. In order to gain increased understanding of how people living with SCD experience daily life, coping with disease, support by health care providers and the demand for new therapies, a patient survey addressing these areas was conducted.
Aims
The aim with this survey was to gain deeper understanding of different aspects of life with SCD by providing a channel for patients to air their own views. The outcome will provide important information and, in combination with future feasibility studies, will guide the design of the first clinical study aimed at treating the early symptoms of pain crises in SCD patients.
Methods
A 29-question survey was created to gather input on a wide variety of topics related to the lives of people living with SCD. This questionnaire was developed by Modus Therapeutics AB, Sweden, in conjunction with Micromattie Consulting Inc., USA. Experts and leaders of community-based organizations participated in two focus group sessions to ensure that the text and structure were ethical and appropriate for the intended purpose. The survey was hosted at www.modustxpatientsurvey.com. Patients answered the survey directly, or had their views entered in by a caregiver. The answers are anonymous. During the initial period, survey promotion occurred within the Sickle Cell Warriors online community and later, additional connections within the network of community-based organizations were leveraged. The survey was open for access during the period of January 10, 2017 through March 1, 2017.
Results
An interim analysis was conducted on January 31, 2017. Basic demographic data is presented in table 1. Responders were located mainly in the US. Medical history related questions indicate that fatigue (40%), aches/pain (37%), irritability (27%) and appetite (20%) are early symptoms and increase just before the onset of a pain crises. However, 7% reported infrequent signs and 19% never experienced an indicator of pain crisis. Patients take initiative at home to manage the onset of an acute crisis and the top 5 home strategies reported were: prescription pain medication (15%), sleep/rest (15%), apply heat using heating pad/blanket/bath/shower (13%), increase fluid intake (12%), and finally avoid stress (9%). Further it is clear, that people living with SCD are motivated to try a new therapy that could provide “significant relief” and “prevent symptoms from happening” due to their SCD.

Conclusion
The survey collected feedback about topics for which the patient is the best source of information. It is obvious that people with SCD are willing to self-medicate by subcutaneous injections and that there is a need for new tools and medications. With support from the answers from the survey, specific aspects will be considered while designing a first clinical study for subcutaneous sevuparin/DF02 administration to treat early symptoms of painful crisis in an at-home setting.
Session topic: 25. Sickle cell disease
Keyword(s): sickle cell disease, Patient, Development, Clinical Trial


