Abstract: PB2144
Type: Publication Only
Background
Older studies have suggested activation of the alternative pathway of complement (APC) in sickle cell disease (SCD). Despite the renewed interest in SCD therapeutics, little is known about APC activation in the clinical setting of SCD, possibly due to the complexity of complement diagnostics.
Aims
We investigated firstly, whether complement activation can be detected in the sera of asymptomatic SCD patients using a simple functional assay, secondly whether it is associated with clinical parameters and thirdly whether it can be blocked in vitro by the complement inhibitor eculizumab.
Methods
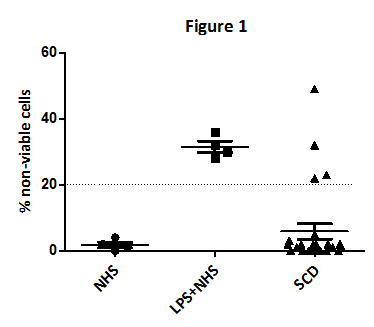
Consecutive asymptomatic SCD patients were enrolled prospectively from November 2016 to January 2017. Patient history, clinical and laboratory data were recorded. Complement activation was detected in patient sera using the modified Ham test, a cell proliferation assay based on the susceptibility of a PNH (paroxysmal nocturnal hemoglobinuria)-like cell line to complement activated serum. Normal human serum (NHS) was used as a negative control and lipopolysaccharides (LPS)-incubated normal serum as a positive control. All samples were tested in triplicates and twice. Eculizumab containing serum (ECU) was collected from a PNH patient within 60 minutes after the infusion and used to test complement blockade by eculizumab in the modified Ham test.
Results
We studied 26 SCD patients (36±11 years of age, 12 male:14 female, 8 HbS/S: 18 HbS/β-Thal). Among them, only 6 had a history of painful crisis and 15 a splenectomy, 15 were on hydroxyurea and 12 on antithrombotic treatment.
Based on previous studies and present controls, percentage of non-viable cells higher than 20% was considered a positive modified Ham test, indicating increased APC activation in 4 SCD patients (Figure 1). Positive patients had significantly increased HbS levels (80.5±11 vs 61.8±16%, p=0.037), while 2/4 were homozygous (HbS/S) and 2/4 heterozygous (HbS/β-Thal). The majority (3/4) were not on hydroxyurea. No significant difference was found regarding age, gender, platelets, white blood cells, Hb, HbF, LDH and bilirubin levels between patients with and without complement activation.
Then, we evaluated in vitro the efficacy of complement inhibition by eculizumab in the modified Ham test. Mixing eculizumab serum (ECU) with complement activated sera demonstrated a dose-killing relationship that was consistent across the 4 patients.
Conclusion
Our results suggest that complement dysregulation is evident in asymptomatic SCD patients with increased HbS levels, an important tool in everyday clinical practice. APC activation during a painful crisis and the role of hydroxyurea need to be further investigated in larger series validating the role of different functional assays. Effective inhibition of complement activation in vitro is promising for future studies in selected patients.
Session topic: 25. Sickle cell disease





