
Contributions
Abstract: PB2117
Type: Publication Only
Background
Stent thrombosis and hemorrhage are the main complications after endovascular procedures for cerebral aneurysm treatment. Identifying an optimal pre-procedure response to antiplatelet therapy is essential to guarantee a successful result. A high variability in the individual responses to the antiagregant effect of aspirin and, specially, with clopidogrel has been reported. The VerifyNow® System (Accumetrics, San Diego, CA, USA) performs a turbidimetric-based optical detection of induced platelet aggregation in response to major antiplatelet agents (P2Y12 inhibitors, aspirin, GP IIb/IIIa inhibitors).
Aims
1) To measure the antiplatelet effect of aspirin and clopidogrel with the point-of-care VerifyNow® assay in patients with brain aneurysms before undergoing endovascular treatment. 2) To compare the results with two alternative methods: impedance aggregometry. and PFA-100.
Methods
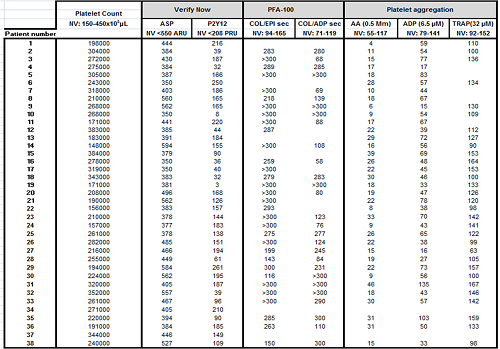
38 patients with cerebral aneurysms, scheduled for elective endovascular procedure, were included in the study. All of them had started taking aspirin at a dose of 100 mg daily and clopidogrel at a dose of 75 mg daily 7 to 10 days before testing aspirin and clopidogrel sensitivity. The following functional tests were performed in all of them before the procedure: 1) VerifyNow® assay: Aspirin Reaction Units (ARU) <550 and P2Y12 Unit Reaction Units (PRU) <208 were considered to be good response to aspirin and clopidogrel respectively. PRU <85 was considered hyper-response to clopidogrel. 2) Impedance aggregometry from whole blood (Multiplate® analyzer, Roche Diagnostics, Mannheim, Germany): arachidonic acid (AA), adenosine diphosphate (ADP) and thrombin receptor activating peptide (TRAP) were used as agonists. TRAP was used to determine baseline platelet function. Aggregation with AA <40 U and aggregation with ADP < 47 U were considered good responses to aspirin and clopidogrel respectively. 3) PFA-100: an overall assessment of platelet function was performed using epinephrine-collagen (COL/EPI) and ADP-collagen (COL/ADP) cartridges. Although COL/ADP is not an appropriate method to evaluate the effect of tienopyridines, we performed it to analyze whether hyper-responders to clopidogrel detected by VerifyNow® were also identified with PFA-100.
Results
The results of platelet function testing with three different methods are summarized in table 1. None of the patients showed thrombocytopenia. Good response to aspirin was observed in 84.21%, 97.36% and 93.75% of the patients using VerifyNow®, Multiplate® and PFA-100 respectively. Good response to clopidogrel was detected in 86.84%, 38.88% and 62.5% of the patients using VerifyNow®, Multiplate® and PFA-100 respectively. VerifyNow® identified 6 (15.78%) aspirin-resistant patients. However, PFA-100 and Multiplate® assays showed a significant aspirin-mediated platelet dysfunction in 5 of them. Low response to clopidogrel was detected by Verifynow® in 5 (13.15%) patients consistent with Multiplate® results. Verifynow® identified 10 patients with excessive response, but only 2 of these results were reproduced by Multiplate® or COL/ADP. Multiplate® detected 19 patients (50%) with suboptimal response to clopidogrel, although these results did not correlate with those obtained by VerifyNow®.
Conclusion
The effect of aspirin can be accurately measured by platelet aggregation and PFA-100 (with COL/EPI); however, VerifyNow® seems to identify a higher number of poor responders. Multiplate® assay using only ADP is not good enough to detect clopidogrel-mediated platelet dysfunction since it is not specific for the P2Y12 receptor. The addition of PGE1 to the ADP test may increase its sensitivity. VerifyNow® assay seems to overestimate the effect of clopidogrel, since hyper-response data are not reproduced by other techniques. According to our results, a high interindividual variability in response to clopidogrel is observed.
Session topic: 32. Platelets disorders
Keyword(s): Surgery, Point-of-care, Aspirin, Anti-platelet therapies
Abstract: PB2117
Type: Publication Only
Background
Stent thrombosis and hemorrhage are the main complications after endovascular procedures for cerebral aneurysm treatment. Identifying an optimal pre-procedure response to antiplatelet therapy is essential to guarantee a successful result. A high variability in the individual responses to the antiagregant effect of aspirin and, specially, with clopidogrel has been reported. The VerifyNow® System (Accumetrics, San Diego, CA, USA) performs a turbidimetric-based optical detection of induced platelet aggregation in response to major antiplatelet agents (P2Y12 inhibitors, aspirin, GP IIb/IIIa inhibitors).
Aims
1) To measure the antiplatelet effect of aspirin and clopidogrel with the point-of-care VerifyNow® assay in patients with brain aneurysms before undergoing endovascular treatment. 2) To compare the results with two alternative methods: impedance aggregometry. and PFA-100.
Methods
38 patients with cerebral aneurysms, scheduled for elective endovascular procedure, were included in the study. All of them had started taking aspirin at a dose of 100 mg daily and clopidogrel at a dose of 75 mg daily 7 to 10 days before testing aspirin and clopidogrel sensitivity. The following functional tests were performed in all of them before the procedure: 1) VerifyNow® assay: Aspirin Reaction Units (ARU) <550 and P2Y12 Unit Reaction Units (PRU) <208 were considered to be good response to aspirin and clopidogrel respectively. PRU <85 was considered hyper-response to clopidogrel. 2) Impedance aggregometry from whole blood (Multiplate® analyzer, Roche Diagnostics, Mannheim, Germany): arachidonic acid (AA), adenosine diphosphate (ADP) and thrombin receptor activating peptide (TRAP) were used as agonists. TRAP was used to determine baseline platelet function. Aggregation with AA <40 U and aggregation with ADP < 47 U were considered good responses to aspirin and clopidogrel respectively. 3) PFA-100: an overall assessment of platelet function was performed using epinephrine-collagen (COL/EPI) and ADP-collagen (COL/ADP) cartridges. Although COL/ADP is not an appropriate method to evaluate the effect of tienopyridines, we performed it to analyze whether hyper-responders to clopidogrel detected by VerifyNow® were also identified with PFA-100.
Results
The results of platelet function testing with three different methods are summarized in table 1. None of the patients showed thrombocytopenia. Good response to aspirin was observed in 84.21%, 97.36% and 93.75% of the patients using VerifyNow®, Multiplate® and PFA-100 respectively. Good response to clopidogrel was detected in 86.84%, 38.88% and 62.5% of the patients using VerifyNow®, Multiplate® and PFA-100 respectively. VerifyNow® identified 6 (15.78%) aspirin-resistant patients. However, PFA-100 and Multiplate® assays showed a significant aspirin-mediated platelet dysfunction in 5 of them. Low response to clopidogrel was detected by Verifynow® in 5 (13.15%) patients consistent with Multiplate® results. Verifynow® identified 10 patients with excessive response, but only 2 of these results were reproduced by Multiplate® or COL/ADP. Multiplate® detected 19 patients (50%) with suboptimal response to clopidogrel, although these results did not correlate with those obtained by VerifyNow®.

Conclusion
The effect of aspirin can be accurately measured by platelet aggregation and PFA-100 (with COL/EPI); however, VerifyNow® seems to identify a higher number of poor responders. Multiplate® assay using only ADP is not good enough to detect clopidogrel-mediated platelet dysfunction since it is not specific for the P2Y12 receptor. The addition of PGE1 to the ADP test may increase its sensitivity. VerifyNow® assay seems to overestimate the effect of clopidogrel, since hyper-response data are not reproduced by other techniques. According to our results, a high interindividual variability in response to clopidogrel is observed.
Session topic: 32. Platelets disorders
Keyword(s): Surgery, Point-of-care, Aspirin, Anti-platelet therapies


