
Contributions
Abstract: PB2072
Type: Publication Only
Background
Diffuse Large B-cell Lymphoma (DLBCL) is a heterogenous disease with variable clinical and pathologic presentations. Using gene expression profiling or Lymph2Cx assay, DLBCL can be assigned as germinal center (GCB) or non-germinal center (Non-GCB) subtype. However such assays remain cumbersome or unavailable for routine clinical care. Immunohistochemical (IHC) algorithms, such as the one proposed by Hans et al., are easy to use tools but demonstrated variable concordance to gene expression profiling. Importantly, cell of origin (COO) assignment appears to influence overall survival (OS) but not progression free survival (PFS). Furthermore, antiapoptotic BCL-2 oncogene expression confers prognostic significance in GCB DLBCL but its significance in Non-GCB is unknown.
Aims
To examine the prognostic impact of cell of origin (COO) assignment in conjunction with BCL-2 expression in a cohort of DLBCL patients.
Methods
After due IRB approval, adult patients diagnosed with DLBCL and treated at our institution between 2010 – 2015 were identified. Clinical and pathologic variables were retrospectively abstracted. IHC expression was deemed positive if > 30% of staining was observed. Cell of origin analysis was determined by the Hans criteria. All patients were treated with combinational chemotherapy containing rituximb. Patients who died prior to receiving therapy were excluded. Categorical and continuous variables were compared using Chi-squared and Wilcoxon tests, respectively. Time to end point analysis was computed using the method of Kaplan and Meier with log ranks. Relapse, progression or death was considered an event for PFS estimation. Analysis was computed using JMP software, version 11.
Results
A total of 122 patients were identified and analyzed. Median follow up of the cohort was 21.8 (1.47 – 107) months, during which OS was 73.5% and PFS was 59.9%. Stratified by IPI, 2-year OS was 85%, 76.3%, 72% and 49.5% for low, low-intermediate, high-intermediate and high risk patients, respectively (p = 0.006). After stratifying patients to GCB and Non-GCB, baseline characteristics between the strata with regards to gender, age, stage, extranodal disease, lactate dehydrogenase (LDH), International Prognostic Index (IPI) and BCL-2 expression were not significantly different.
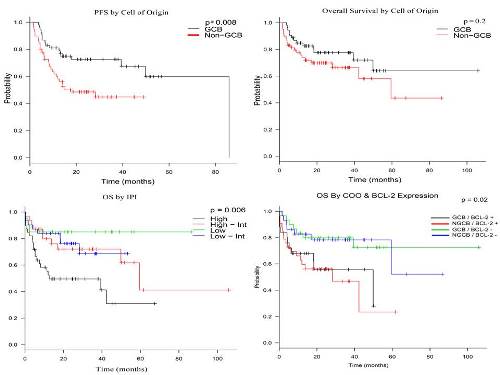
Conclusion
COO assignment using IHC demonstrated superior PFS for GCB over non-GCB however this was mitigated by BCL-2 expression. This raises questions regarding the currently presumed pathogenesis of the different subtypes and how to utilize the currently available targeted therapies including BCL-2 inhibitors. These observations warrant further study.
Session topic: 18. Non-Hodgkin & Hodgkin lymphoma - Biology
Keyword(s): Germinal center, Diffuse large B cell lymphoma, BCL2
Abstract: PB2072
Type: Publication Only
Background
Diffuse Large B-cell Lymphoma (DLBCL) is a heterogenous disease with variable clinical and pathologic presentations. Using gene expression profiling or Lymph2Cx assay, DLBCL can be assigned as germinal center (GCB) or non-germinal center (Non-GCB) subtype. However such assays remain cumbersome or unavailable for routine clinical care. Immunohistochemical (IHC) algorithms, such as the one proposed by Hans et al., are easy to use tools but demonstrated variable concordance to gene expression profiling. Importantly, cell of origin (COO) assignment appears to influence overall survival (OS) but not progression free survival (PFS). Furthermore, antiapoptotic BCL-2 oncogene expression confers prognostic significance in GCB DLBCL but its significance in Non-GCB is unknown.
Aims
To examine the prognostic impact of cell of origin (COO) assignment in conjunction with BCL-2 expression in a cohort of DLBCL patients.
Methods
After due IRB approval, adult patients diagnosed with DLBCL and treated at our institution between 2010 – 2015 were identified. Clinical and pathologic variables were retrospectively abstracted. IHC expression was deemed positive if > 30% of staining was observed. Cell of origin analysis was determined by the Hans criteria. All patients were treated with combinational chemotherapy containing rituximb. Patients who died prior to receiving therapy were excluded. Categorical and continuous variables were compared using Chi-squared and Wilcoxon tests, respectively. Time to end point analysis was computed using the method of Kaplan and Meier with log ranks. Relapse, progression or death was considered an event for PFS estimation. Analysis was computed using JMP software, version 11.
Results
A total of 122 patients were identified and analyzed. Median follow up of the cohort was 21.8 (1.47 – 107) months, during which OS was 73.5% and PFS was 59.9%. Stratified by IPI, 2-year OS was 85%, 76.3%, 72% and 49.5% for low, low-intermediate, high-intermediate and high risk patients, respectively (p = 0.006). After stratifying patients to GCB and Non-GCB, baseline characteristics between the strata with regards to gender, age, stage, extranodal disease, lactate dehydrogenase (LDH), International Prognostic Index (IPI) and BCL-2 expression were not significantly different.

Conclusion
COO assignment using IHC demonstrated superior PFS for GCB over non-GCB however this was mitigated by BCL-2 expression. This raises questions regarding the currently presumed pathogenesis of the different subtypes and how to utilize the currently available targeted therapies including BCL-2 inhibitors. These observations warrant further study.
Session topic: 18. Non-Hodgkin & Hodgkin lymphoma - Biology
Keyword(s): Germinal center, Diffuse large B cell lymphoma, BCL2


