
Contributions
Abstract: PB2059
Type: Publication Only
Background
Treatment with the Janus-activated kinase (JAK) 1 and 2 inhibitor ruxolitinib decreases constitutional symptoms and spleen size in myelofibrosis.
Aims
In our retrospective study we analysed myelofibrosis patients treated with Ruxolitinib and cytoreductive treatment with Hydroxiurea and supportive therapy followed in our Department from 2012 to 2016 to evaluate rate of infections developed.
Methods
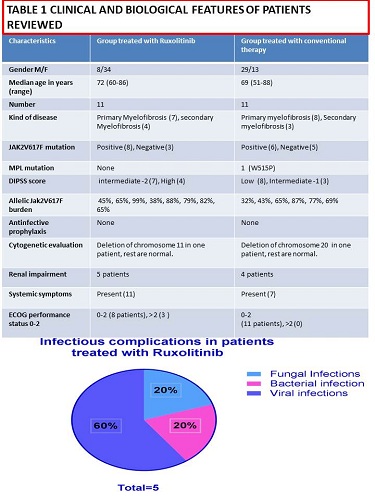
We reviewed 22 patients presenting myelofibrosis (median age 72, range 60-86)describing clinical and biological features (Table 1). Our aim was description of documented infections identified with conventional treatment and with Ruxolitinib. They were 11 treated with JAK inhibitors and 11 with Hydroxiurea taken orally, similar for age and clinical features.
Results
A total of 22 patients consecutively diagnosed were included in this analysis. There were 15 primary and 7 secondary myelofibrosis patients. According to the Dynamic International Prognostic Scoring System (DIPSS) 8 were low risk, 10 were intermediate risk and 4 were high.
Conclusion
These data in our small series of patients suggest a higher incidence of ruxolitinib associated infections observed in clinical practice compared to traditional treatment. Immunosuppressive effect of Ruxolitinib is reported and the use of this drug in the transplant setting with beneficial effects on alloreactivity and on graft versus host disease is becoming more common.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Myelofibrosis, Janus Kinase inhibitor, Immune deficiency
Abstract: PB2059
Type: Publication Only
Background
Treatment with the Janus-activated kinase (JAK) 1 and 2 inhibitor ruxolitinib decreases constitutional symptoms and spleen size in myelofibrosis.
Aims
In our retrospective study we analysed myelofibrosis patients treated with Ruxolitinib and cytoreductive treatment with Hydroxiurea and supportive therapy followed in our Department from 2012 to 2016 to evaluate rate of infections developed.
Methods
We reviewed 22 patients presenting myelofibrosis (median age 72, range 60-86)describing clinical and biological features (Table 1). Our aim was description of documented infections identified with conventional treatment and with Ruxolitinib. They were 11 treated with JAK inhibitors and 11 with Hydroxiurea taken orally, similar for age and clinical features.
Results
A total of 22 patients consecutively diagnosed were included in this analysis. There were 15 primary and 7 secondary myelofibrosis patients. According to the Dynamic International Prognostic Scoring System (DIPSS) 8 were low risk, 10 were intermediate risk and 4 were high.

Conclusion
These data in our small series of patients suggest a higher incidence of ruxolitinib associated infections observed in clinical practice compared to traditional treatment. Immunosuppressive effect of Ruxolitinib is reported and the use of this drug in the transplant setting with beneficial effects on alloreactivity and on graft versus host disease is becoming more common.
Session topic: 16. Myeloproliferative neoplasms - Clinical
Keyword(s): Myelofibrosis, Janus Kinase inhibitor, Immune deficiency


