CARFILZOMIB-LENALIDOMIDE-DEXAMETHASONE IN THE MANAGEMENT OF RELAPSED AND REFRACTORY MULTIPLE MYELOMA: A REAL-LIFE EXPERIENCE
(Abstract release date: 05/18/17)
EHA Library. Cerchione C. 05/18/17; 182734; PB2020

Dr. Claudio Cerchione
Contributions
Contributions
Abstract
Abstract: PB2020
Type: Publication Only
Background
Carfilzomib is an epoxyketone proteasome inhibitor of second generation, proved to be effective in relapsed and refractory Multiple Myeloma (rrMM).
Aims
In this retrospective observational trial, it has been evaluated efficacy and tolerance of Carfilzomib, in combination with lenalidomide-dexamethasone (KRD) as salvage regimen in patients with relapsed and refractory MM (rrMM), whose prognosis is particularly severe.
Methods
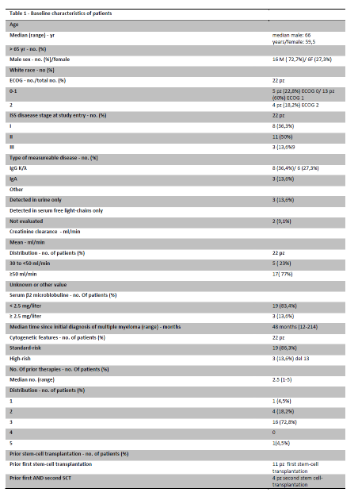
21 patients (12 M/9 F, Table 1), with rrMM, median age at diagnosis 62 years (r. 47-75), median age at start of treatment 65 years (r. 53-81) treated with several lines of treatments (median 3, r. 2-10), included 2 patients refractory to Bortezomib, underwent to KRD regimen (ASPIRE trial schedule: Carfilzomib starting dose 20 mg/sqm on days 1,2 of cycle 1, target dose 27 mg/sqm thereafter; Lenalidomide 25 mg on days 1 through 21; Dexamethasone 40 mg on days 1,8,15 and 22, every 28 days) for a median treatment cycles of 2 (r 1-8) .
ISS was equally distributed, and cytogenetic was evaluable in 8 patients, and in particular one del13q14 1qgain, one del 13q14 and one t(11;14). 86% of patients had previously been treated with schedule containing bortezomib and IMIDs, and 33% had also received radiotherapy. 57% of them had undergone at least to a single auSCT.
Results
Carfilzomib was well tolerated, with grade 2 anemia in 28% of patients, without necessity of blood transfusions; 5% grade 1 and 9.5% grade 3 neutropenia (no ospedalization was required, no septic shocks were observed); 33% grade 2, 19% grade 3 and 5% grade 4 thrombocytopenia, without hemorrhagic events and necessity of transufsions. Concerning severe extrahematologic toxicity, it was observed grade 1 pneumonia in 47 % of patients, treated by common antibiotic drugs; grade 2 Hypertension in 24 % of patients; grade 3 arrhythmias in 5% of patients; grade 2 dyspnea in 5% of patients; grade 1 fatigue in 9.5% of patients.
According to IMWG criteria, after a median follow-up of 3 months (r.1-13), ORR was 66,7% (14/21 : 8 VGPR, 6 PR) with 3 progressive diseases and 2 patients in stable disease, which can be considered as an impressive result in this subset of rrMM patients. In particular, for 1 patient, KRD was, after having achieved at least a PR, a bridge to second auSCT.
Median time to response was 2 months (r.1-4), median OS from diagnosis was 47 months (9-170 range), median OS from start of Carfilzomib was 3 months (range 1-13).
Conclusion
KRD has shown significant efficacy in a particularly severe setting of patients, relapsed and refractory to all available therapeutic resources, and, in particular cases, it could be considered as a bridge to a second autologous or allogenic SCT.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Relapse, Proteasome inhibitor, Myeloma, Imids
Abstract: PB2020
Type: Publication Only
Background
Carfilzomib is an epoxyketone proteasome inhibitor of second generation, proved to be effective in relapsed and refractory Multiple Myeloma (rrMM).
Aims
In this retrospective observational trial, it has been evaluated efficacy and tolerance of Carfilzomib, in combination with lenalidomide-dexamethasone (KRD) as salvage regimen in patients with relapsed and refractory MM (rrMM), whose prognosis is particularly severe.
Methods
21 patients (12 M/9 F, Table 1), with rrMM, median age at diagnosis 62 years (r. 47-75), median age at start of treatment 65 years (r. 53-81) treated with several lines of treatments (median 3, r. 2-10), included 2 patients refractory to Bortezomib, underwent to KRD regimen (ASPIRE trial schedule: Carfilzomib starting dose 20 mg/sqm on days 1,2 of cycle 1, target dose 27 mg/sqm thereafter; Lenalidomide 25 mg on days 1 through 21; Dexamethasone 40 mg on days 1,8,15 and 22, every 28 days) for a median treatment cycles of 2 (r 1-8) .
ISS was equally distributed, and cytogenetic was evaluable in 8 patients, and in particular one del13q14 1qgain, one del 13q14 and one t(11;14). 86% of patients had previously been treated with schedule containing bortezomib and IMIDs, and 33% had also received radiotherapy. 57% of them had undergone at least to a single auSCT.
Results
Carfilzomib was well tolerated, with grade 2 anemia in 28% of patients, without necessity of blood transfusions; 5% grade 1 and 9.5% grade 3 neutropenia (no ospedalization was required, no septic shocks were observed); 33% grade 2, 19% grade 3 and 5% grade 4 thrombocytopenia, without hemorrhagic events and necessity of transufsions. Concerning severe extrahematologic toxicity, it was observed grade 1 pneumonia in 47 % of patients, treated by common antibiotic drugs; grade 2 Hypertension in 24 % of patients; grade 3 arrhythmias in 5% of patients; grade 2 dyspnea in 5% of patients; grade 1 fatigue in 9.5% of patients.
According to IMWG criteria, after a median follow-up of 3 months (r.1-13), ORR was 66,7% (14/21 : 8 VGPR, 6 PR) with 3 progressive diseases and 2 patients in stable disease, which can be considered as an impressive result in this subset of rrMM patients. In particular, for 1 patient, KRD was, after having achieved at least a PR, a bridge to second auSCT.
Median time to response was 2 months (r.1-4), median OS from diagnosis was 47 months (9-170 range), median OS from start of Carfilzomib was 3 months (range 1-13).

Conclusion
KRD has shown significant efficacy in a particularly severe setting of patients, relapsed and refractory to all available therapeutic resources, and, in particular cases, it could be considered as a bridge to a second autologous or allogenic SCT.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Relapse, Proteasome inhibitor, Myeloma, Imids
{{ help_message }}
{{filter}}


