DEPP RESPONCES WITH CARLFIZOMIB-LENALIDOMIDE-DEXAMETHASONE IN RELAPSED/REFRATORY MULTIPLE MYELOMA PATIENTS: A REAL LIFE EXPERIENCE
(Abstract release date: 05/18/17)
EHA Library. Pavone V. 05/18/17; 182733; PB2019

Dr. Vincenzo Pavone
Contributions
Contributions
Abstract
Abstract: PB2019
Type: Publication Only
Background
Carfilzomib is a new proteasome inibitor with in contrast to the reversible binding of bortezomib, binds irreversibly and selectively to its target: the chymotrypsin-like activity of the 20S proteasome. The phase IB/II PX-171-006 study was the first study in which carfilzomib was combined with lenalidomide and dexamethasone. In the phase I dose-escalation part the maximum planned dose was established as well tolerated and in the phase II part the study focused the efficacy and toxicity in the subgroup treated with maximum planned dose. The ASPIRE trial showed superior response rates and progression free survival for carfilzomib-lenalidomide-dexamethasone compared with lenalidomide-dexamethasone in relapsed/refractory Multiple Myeloma patients.
Aims
The aims is explorer the efficacy and tolerability of carfilzomib-lenalidomide-dexamethasone in relapsed/refractory Multiple Myeloma patients in real life.
Methods
All patients received carlfilzomib 20/27 mg/m2 days 1,2,8,9,15 and 16; lenalidomide 25 mg days 1-21 and daxamethasone 20 mg days 1,2,8,9,15,16, 22 and 23, according to post approval access protocol. After 2, 4, 6 and 8 cycles the responses, disease progression and toxicity were assessed using the International Myeloma Working Group Uniform Response Criteria and WHO score respectively.
Results
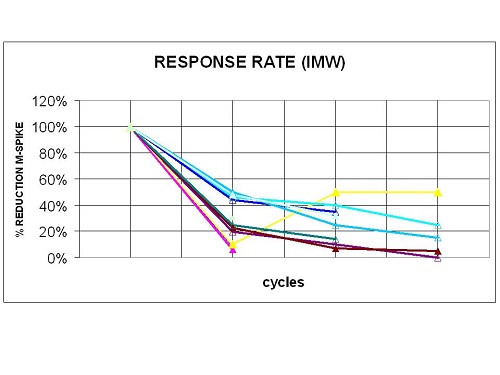
From January 2016 to February 2017 in hematology 'Cardinale G.Panico Hospital' and 'Bari Policlinico', treated 15 relapsed/refractory Multiple Myeloma patients with carfilzomib-lenalidomiode-dexamethasone. Six patients male (40%), 9 female (90%), mean of age 62 years (range 38-79); 10 (66%) and 5 (34%) relapsed/refractory multiple myeloma respectively. Median time from diagnosis to carfilzomib-lenalidomiode-dexamethasone was 46 months (range 12-92); median of prior therapy was 3 (range 1-6); 9 (60%) received autologous transplantation while 1 (6%) allogeneic; 11 (73%) prior therapy with lenalidomide; 15 (100%) prior therapy with borthezomib; 2 (14%) prior therapy with pomalidomide (table 1). Eleven (73%) patients achieved after 2 cycles a response rate ≥ PR, of these 3 VGPR. After 4 cycles, 5 (33%) and 1 (7%) have obtained at least a VGPR and CR respectively (Fig 1). Three patients were not evaluated for treatment discontinuation because of rapid progression disease and died during first cycle with a median of 5 prior lines therapy. Most grade 3-4 adverse events were haematological and well manageable, 10 (80%) trombocitopenia and 5 (35%) neutropenia grado 3-4. Dyspnea, fatigue and pyrexia were higher but were mostly grades 1 and 2. Only 2 patients developed respiratory failure and pneumonia while cardiac failure, ischemic heart disease and hypertension not were detected. Table.1; Baseline patient characteristics
MEAN OF AGE, years (range) | 62 (38-79) |
MULTIPLE MYELOMA, n (%)
| 10 (66) 5 (34) |
MULTIPLE MYELOMA SUBGROUP, n (%)
| 6 (40) 2 (14) 7 (46) |
STAGING, n (%) DURIE-SALMON
ISS
| 3 (20) 12 (80) 7 (47) 8 (53) |
MEDIAN TIME FROM DIAGNOSIS TO KRD, months (range) | 46 (12-92) |
MEDIAN OF PRIOR THERAPY, lines (range) | 3 (1-6) |
PRIOR TRASPLANT, n (%)
| 9 (60) 1 (6) |
PRIOR THERAPY, n (%)
| 11 (73) 15 (100) 2 (14) |
Conclusion
Carfilzomib-lenalidomide-dexamethasone is a powerful and efficacy association in relapsed/refractory Multiple Myeloma patients, which allows the achievement of deep responses from the first cycle of therapy. Non haematological adverse events of grade 3 or higher were reported in only 2 patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Relapse, Multiple Myeloma
Abstract: PB2019
Type: Publication Only
Background
Carfilzomib is a new proteasome inibitor with in contrast to the reversible binding of bortezomib, binds irreversibly and selectively to its target: the chymotrypsin-like activity of the 20S proteasome. The phase IB/II PX-171-006 study was the first study in which carfilzomib was combined with lenalidomide and dexamethasone. In the phase I dose-escalation part the maximum planned dose was established as well tolerated and in the phase II part the study focused the efficacy and toxicity in the subgroup treated with maximum planned dose. The ASPIRE trial showed superior response rates and progression free survival for carfilzomib-lenalidomide-dexamethasone compared with lenalidomide-dexamethasone in relapsed/refractory Multiple Myeloma patients.
Aims
The aims is explorer the efficacy and tolerability of carfilzomib-lenalidomide-dexamethasone in relapsed/refractory Multiple Myeloma patients in real life.
Methods
All patients received carlfilzomib 20/27 mg/m2 days 1,2,8,9,15 and 16; lenalidomide 25 mg days 1-21 and daxamethasone 20 mg days 1,2,8,9,15,16, 22 and 23, according to post approval access protocol. After 2, 4, 6 and 8 cycles the responses, disease progression and toxicity were assessed using the International Myeloma Working Group Uniform Response Criteria and WHO score respectively.
Results
From January 2016 to February 2017 in hematology 'Cardinale G.Panico Hospital' and 'Bari Policlinico', treated 15 relapsed/refractory Multiple Myeloma patients with carfilzomib-lenalidomiode-dexamethasone. Six patients male (40%), 9 female (90%), mean of age 62 years (range 38-79); 10 (66%) and 5 (34%) relapsed/refractory multiple myeloma respectively. Median time from diagnosis to carfilzomib-lenalidomiode-dexamethasone was 46 months (range 12-92); median of prior therapy was 3 (range 1-6); 9 (60%) received autologous transplantation while 1 (6%) allogeneic; 11 (73%) prior therapy with lenalidomide; 15 (100%) prior therapy with borthezomib; 2 (14%) prior therapy with pomalidomide (table 1). Eleven (73%) patients achieved after 2 cycles a response rate ≥ PR, of these 3 VGPR. After 4 cycles, 5 (33%) and 1 (7%) have obtained at least a VGPR and CR respectively (Fig 1). Three patients were not evaluated for treatment discontinuation because of rapid progression disease and died during first cycle with a median of 5 prior lines therapy. Most grade 3-4 adverse events were haematological and well manageable, 10 (80%) trombocitopenia and 5 (35%) neutropenia grado 3-4. Dyspnea, fatigue and pyrexia were higher but were mostly grades 1 and 2. Only 2 patients developed respiratory failure and pneumonia while cardiac failure, ischemic heart disease and hypertension not were detected. Table.1; Baseline patient characteristics
MEAN OF AGE, years (range) | 62 (38-79) |
MULTIPLE MYELOMA, n (%)
| 10 (66) 5 (34) |
MULTIPLE MYELOMA SUBGROUP, n (%)
| 6 (40) 2 (14) 7 (46) |
STAGING, n (%) DURIE-SALMON
ISS
| 3 (20) 12 (80) 7 (47) 8 (53) |
MEDIAN TIME FROM DIAGNOSIS TO KRD, months (range) | 46 (12-92) |
MEDIAN OF PRIOR THERAPY, lines (range) | 3 (1-6) |
PRIOR TRASPLANT, n (%)
| 9 (60) 1 (6) |
PRIOR THERAPY, n (%)
| 11 (73) 15 (100) 2 (14) |

Conclusion
Carfilzomib-lenalidomide-dexamethasone is a powerful and efficacy association in relapsed/refractory Multiple Myeloma patients, which allows the achievement of deep responses from the first cycle of therapy. Non haematological adverse events of grade 3 or higher were reported in only 2 patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Relapse, Multiple Myeloma
{{ help_message }}
{{filter}}


