
Contributions
Abstract: PB2014
Type: Publication Only
Background
Lenalidomide is an oral immunomodulatory medication with clinical efficacy in relapsed/refractory and treatment naïve multiple myeloma (MM), 5q- myelodysplasia and lymphoma. Lenalidomide is eliminated predominantly unchanged by urinary excretion. Renal impairment is common in MM (15-40%) and approximately 10% of MM requires dialysis. However, there is a paucity of clinical safety data of Lenalidomide in ESRF.
Aims
To provide real-world evidence of an institutional experience of the use of Lenalidomide in dialysis-dependent MM.
Methods
We performed a retrospective audit of our in-centre experience with treating dialysis-dependent MM with Lenalidomide and included patients who completed at least one cycle of therapy. Patients were assessed for haematological toxicity, significant infective complications, thrombosis, disease response and progression-free survival. Best response was stratified by IMWG criteria. Patients’ baseline characteristics, prior therapies, cytogenetics and FISH data were collected.
Results
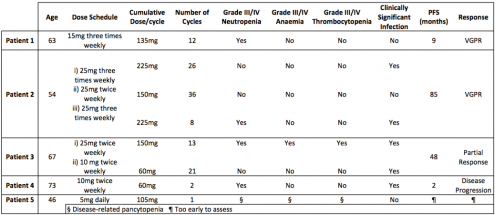
We identified 5 patients treated between 2010 and 2017, aged between 54 to 73 years old. All patients had relapsed/refractory MM and dialysis dependent ESRF. The median number of prior therapies was two. One patient had t(11,14) on FISH and died from progressive disease. Dose schedules are shown in the table. Almost all patients experienced grade III-IV haematological toxicity and 60% had grade III-IV infection. There was a positive correlation between dose and toxicity, and furthermore there appeared to be an inverse relationship between age and tolerated dose. Haematological toxicities and infection were ameliorated by dose adjustment in most instances. There was no drug related mortality, however one patient died of progressive disease. Four of the five patients were prescribed aspirin thromboprophylaxis, with no proven thrombotic complications seen.
Conclusion
Our experience builds on the emerging evidence that reduced dose of Lenalidomide can be safely prescribed for dialysis-dependent MM, with clinical efficacy. In our cohort most patients took Lenalidomide on days of dialysis only. There was significant variation of dose-related tolerability between patients. However, toxicity was manageable with diligent monitoring and dose adjustment.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Renal failure, Myeloma, Immunomodulatory thalidomide analog, Dose intensity
Abstract: PB2014
Type: Publication Only
Background
Lenalidomide is an oral immunomodulatory medication with clinical efficacy in relapsed/refractory and treatment naïve multiple myeloma (MM), 5q- myelodysplasia and lymphoma. Lenalidomide is eliminated predominantly unchanged by urinary excretion. Renal impairment is common in MM (15-40%) and approximately 10% of MM requires dialysis. However, there is a paucity of clinical safety data of Lenalidomide in ESRF.
Aims
To provide real-world evidence of an institutional experience of the use of Lenalidomide in dialysis-dependent MM.
Methods
We performed a retrospective audit of our in-centre experience with treating dialysis-dependent MM with Lenalidomide and included patients who completed at least one cycle of therapy. Patients were assessed for haematological toxicity, significant infective complications, thrombosis, disease response and progression-free survival. Best response was stratified by IMWG criteria. Patients’ baseline characteristics, prior therapies, cytogenetics and FISH data were collected.
Results
We identified 5 patients treated between 2010 and 2017, aged between 54 to 73 years old. All patients had relapsed/refractory MM and dialysis dependent ESRF. The median number of prior therapies was two. One patient had t(11,14) on FISH and died from progressive disease. Dose schedules are shown in the table. Almost all patients experienced grade III-IV haematological toxicity and 60% had grade III-IV infection. There was a positive correlation between dose and toxicity, and furthermore there appeared to be an inverse relationship between age and tolerated dose. Haematological toxicities and infection were ameliorated by dose adjustment in most instances. There was no drug related mortality, however one patient died of progressive disease. Four of the five patients were prescribed aspirin thromboprophylaxis, with no proven thrombotic complications seen.

Conclusion
Our experience builds on the emerging evidence that reduced dose of Lenalidomide can be safely prescribed for dialysis-dependent MM, with clinical efficacy. In our cohort most patients took Lenalidomide on days of dialysis only. There was significant variation of dose-related tolerability between patients. However, toxicity was manageable with diligent monitoring and dose adjustment.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Renal failure, Myeloma, Immunomodulatory thalidomide analog, Dose intensity


