
Contributions
Abstract: PB2009
Type: Publication Only
Background
The multiple myeloma (MM) treatment (Tx) landscape is rapidly evolving, with varying Tx practice patterns and access schemes across countries. However real-world (RW) data describing patient (pt) management, MM Tx use and outcomes in some Eastern European Countries are limited.
Aims
To understand the characteristics, management, Tx patterns and outcomes of pts with symptomatic MM in a RW setting in Bulgaria (BG), Croatia (HR) and Slovakia (SK).
Methods
Data were collected within a cross-sectional (X) and retrospective (R) phase of a chart review in 6 countries between June/15 and June/16 by (onco-)hematologists who managed at least 15 pts with MM per month (mo) and were responsible for initiating MM Tx. Data from 3 countries with limited access to MM Tx are shown. In the X-phase, data included characteristics and current Tx by line of therapy for all pts with MM seen during a 3-week observation period, regardless of pts’ Tx status and strategy. In the R-phase, data included pt and disease characteristics at diagnosis, Tx response, comorbidities and clinical outcomes by Tx line. Pts were selected in reverse chronological order and those who had completed specific lines of active Tx within the past 3 mo were included as follows: 3 pts in first line (1L), 4 pts in second-line (2L) and 7 pts in third or higher lines. Analyses were descriptive.
Results
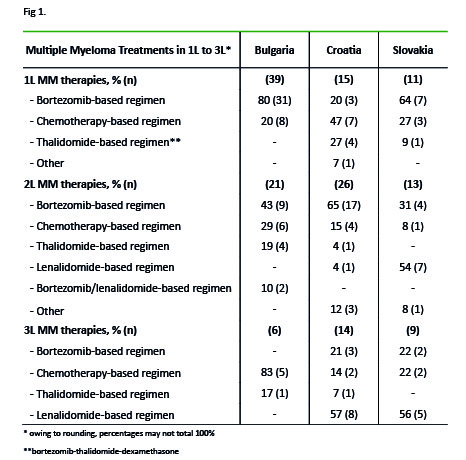
In the X-phase, 7 physicians from BG, 6 from HR and 5 from SK included 84, 89 and 56 pts respectively. In BG, HR and SK respectively, 45%, 51% and 52% of pts were <65 years; 41%, 35% and 38% were 65–75 years. Only 4 pts (from BG) were enrolled in clinical trials. Median time since diagnosis was 24, 31 and 54 mo in BG, HR and SK respectively. In BG, fewer pts received stem cell transplantation (SCT) than in HR and SK (8% vs 24% and 36%). The proportion of pts that had received SCT at any point increased from 1L to 2L (3% to 19%, 7% to 35% and 9% to 54% in BG, HR and SK respectively). 82% of pts in BG, and 70% both in HR and SK were currently receiving Tx (Fig. 1), while 17%, 30% and 25% of pts respectively, were treated previously. Only 4 pts (1 in BG and 3 in SK) had never been treated. In the R-phase, 6 physicians from BG, and 5 from each of HR and SK included 43, 39 and 44 pts respectively. In BG, HR and SK respectively, 44%, 41% and 41% of pts were <65 years; 49%, 36% and 39% were 65–75 years. Depth of response, as assessed by physicians, decreased in BG with each additional line of Tx, but remained stable or increased in HR and SK: 43%, 55% and 50% of pts achieved at least a very good partial response (≥VGPR) in 1L, while 13%, 54% and 69% of pts achieved ≥VGPR in 2L. The most common (≥20%) adverse events (AEs) and comorbidities in 1L were anemia (23% in HR, 43% in SK) and neutropenia (43%) and thrombocytopenia in SK (21%). Mostly, these AEs did not impact on Tx.
Conclusion
These findings suggest a high unmet need for access to more effective and innovative Tx options with manageable safety profiles in these countries. In particular, in BG where bortezomib- and chemotherapy-based regimens are the only treatments used, pts might be re-treated with the same agents, which may explain why most do not achieve ≥VGPR from 2L. In HR and SK, sustained or increased rates of ≥VGPR in 2L may be due to the use of newer or different agents from those used in 1L and to the fact that most pts had previously received a SCT. These RW data provide useful input for economic evaluations of new MM agents to include in earlier Tx lines in these countries.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma
Abstract: PB2009
Type: Publication Only
Background
The multiple myeloma (MM) treatment (Tx) landscape is rapidly evolving, with varying Tx practice patterns and access schemes across countries. However real-world (RW) data describing patient (pt) management, MM Tx use and outcomes in some Eastern European Countries are limited.
Aims
To understand the characteristics, management, Tx patterns and outcomes of pts with symptomatic MM in a RW setting in Bulgaria (BG), Croatia (HR) and Slovakia (SK).
Methods
Data were collected within a cross-sectional (X) and retrospective (R) phase of a chart review in 6 countries between June/15 and June/16 by (onco-)hematologists who managed at least 15 pts with MM per month (mo) and were responsible for initiating MM Tx. Data from 3 countries with limited access to MM Tx are shown. In the X-phase, data included characteristics and current Tx by line of therapy for all pts with MM seen during a 3-week observation period, regardless of pts’ Tx status and strategy. In the R-phase, data included pt and disease characteristics at diagnosis, Tx response, comorbidities and clinical outcomes by Tx line. Pts were selected in reverse chronological order and those who had completed specific lines of active Tx within the past 3 mo were included as follows: 3 pts in first line (1L), 4 pts in second-line (2L) and 7 pts in third or higher lines. Analyses were descriptive.
Results
In the X-phase, 7 physicians from BG, 6 from HR and 5 from SK included 84, 89 and 56 pts respectively. In BG, HR and SK respectively, 45%, 51% and 52% of pts were <65 years; 41%, 35% and 38% were 65–75 years. Only 4 pts (from BG) were enrolled in clinical trials. Median time since diagnosis was 24, 31 and 54 mo in BG, HR and SK respectively. In BG, fewer pts received stem cell transplantation (SCT) than in HR and SK (8% vs 24% and 36%). The proportion of pts that had received SCT at any point increased from 1L to 2L (3% to 19%, 7% to 35% and 9% to 54% in BG, HR and SK respectively). 82% of pts in BG, and 70% both in HR and SK were currently receiving Tx (Fig. 1), while 17%, 30% and 25% of pts respectively, were treated previously. Only 4 pts (1 in BG and 3 in SK) had never been treated. In the R-phase, 6 physicians from BG, and 5 from each of HR and SK included 43, 39 and 44 pts respectively. In BG, HR and SK respectively, 44%, 41% and 41% of pts were <65 years; 49%, 36% and 39% were 65–75 years. Depth of response, as assessed by physicians, decreased in BG with each additional line of Tx, but remained stable or increased in HR and SK: 43%, 55% and 50% of pts achieved at least a very good partial response (≥VGPR) in 1L, while 13%, 54% and 69% of pts achieved ≥VGPR in 2L. The most common (≥20%) adverse events (AEs) and comorbidities in 1L were anemia (23% in HR, 43% in SK) and neutropenia (43%) and thrombocytopenia in SK (21%). Mostly, these AEs did not impact on Tx.

Conclusion
These findings suggest a high unmet need for access to more effective and innovative Tx options with manageable safety profiles in these countries. In particular, in BG where bortezomib- and chemotherapy-based regimens are the only treatments used, pts might be re-treated with the same agents, which may explain why most do not achieve ≥VGPR from 2L. In HR and SK, sustained or increased rates of ≥VGPR in 2L may be due to the use of newer or different agents from those used in 1L and to the fact that most pts had previously received a SCT. These RW data provide useful input for economic evaluations of new MM agents to include in earlier Tx lines in these countries.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma


