
Contributions
Abstract: PB2006
Type: Publication Only
Background
Aims
To define influence of induction therapy regimens, HDT with ASCT to the frequency of Minimal Residual Disease (MRD) negative status and estimate a role MRD in duration of Progression Free Survival (PFS) in multiple myeloma (MM) patients.
Methods
We analyzed 52 patients with MM (median age 55 years, male/female – 2.1:1). The induction therapy with Bortezomib-based regimens (VD, CVD, VMP, PAD) was used in 36/52 (69%) patients, Immunomodulator-based regimens (Thal+D, RD, VRD, PomD) – in 14/52 (27%), chemotherapy – in 2/52 (4%). ASCT is carried out 31 (59.6%) patients. Primary tumor cells phenotype and MRD were detected by 5-color flow cytometry. Clonal plasmatic cells were detected by markers: CD38, CD138, CD45, CD19, CD20, CD27, CD56 and CD117. MRD-negative status considered in identifying less than 1 tumor cell in 10000 (0.01%).
Results
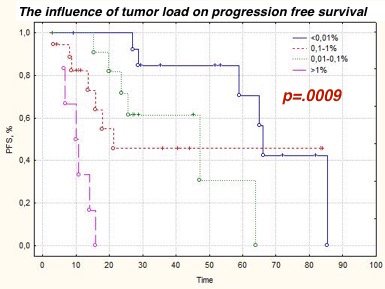
MRD-negative CR was reached in 23.8% (10/42) patients after 4-6 cycles of therapy. The frequency of MRD-negative status in the 'Bortezomib group' was 31% (9/29), in the 'Immunomodulator group' - 7.7% (1/13) (Chi-square =0.1; p >.05). The general frequency of MRD-negative CR after HTD with ASCT was 33.3% (7/21). The carrying out HTD with ASCT allowed to MRD eradication in 36.4% (4/11) patients. One patient with a 'light chain' myeloma lost MRD-negative CR after HTD with ASCT that led to development of a clinical relapse after 6 months. Carrying out a maintenance therapy with bortezomib or lenalidomide didn't allow to achieve MRD-negative status. Carrying out a maintenance therapy with bortezomib or lenalidomide didn't allow to achieve MRD-negative status in patients with MRD-positive response. On the contrary, achievement MRD-negative status promoted to increase of PFS. The PFS median in MRD-positive group of patients (n=36: 21 CR, 6 VGPR, 9 PR) was 21 months, in the MRD-negative group (n=16) – 66 months (p=.0068). The PFS median patients with CR was higher in the MRD-negative group than in the MRD-positive group (66 and 48 months, respectively, p=.0045). The tumor load is also a strong prognostic factor like MRD status. Patients who attained low-level MRD had of benefit in the duration of PFS: ˂0.01% - 66 months, 0.01%>0.1% - 48 months at, 0.1%>1% - 22 months, ˃1% - 10 months (p=.0009).
Conclusion
The frequency of achievement MRD-negative doesn’t depend from program of induction therapy, HDT with ASCT and maintenance therapy. Negative prognostic role of MRD status independent from clinical response. Presence of MRD after treatment to associated with decrease of PFS and early relapse. Control of MRD allows to increase of PFS and can be done by means of modern drugs and its combinations, HDT with ASCT and maintenance therapy. Impact of MRD requires further studies, especially after HDT with ASCT.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Survival, Stem cell transplant, Myeloma, Minimal residual disease (MRD)
Abstract: PB2006
Type: Publication Only
Background
Aims
To define influence of induction therapy regimens, HDT with ASCT to the frequency of Minimal Residual Disease (MRD) negative status and estimate a role MRD in duration of Progression Free Survival (PFS) in multiple myeloma (MM) patients.
Methods
We analyzed 52 patients with MM (median age 55 years, male/female – 2.1:1). The induction therapy with Bortezomib-based regimens (VD, CVD, VMP, PAD) was used in 36/52 (69%) patients, Immunomodulator-based regimens (Thal+D, RD, VRD, PomD) – in 14/52 (27%), chemotherapy – in 2/52 (4%). ASCT is carried out 31 (59.6%) patients. Primary tumor cells phenotype and MRD were detected by 5-color flow cytometry. Clonal plasmatic cells were detected by markers: CD38, CD138, CD45, CD19, CD20, CD27, CD56 and CD117. MRD-negative status considered in identifying less than 1 tumor cell in 10000 (0.01%).
Results
MRD-negative CR was reached in 23.8% (10/42) patients after 4-6 cycles of therapy. The frequency of MRD-negative status in the 'Bortezomib group' was 31% (9/29), in the 'Immunomodulator group' - 7.7% (1/13) (Chi-square =0.1; p >.05). The general frequency of MRD-negative CR after HTD with ASCT was 33.3% (7/21). The carrying out HTD with ASCT allowed to MRD eradication in 36.4% (4/11) patients. One patient with a 'light chain' myeloma lost MRD-negative CR after HTD with ASCT that led to development of a clinical relapse after 6 months. Carrying out a maintenance therapy with bortezomib or lenalidomide didn't allow to achieve MRD-negative status. Carrying out a maintenance therapy with bortezomib or lenalidomide didn't allow to achieve MRD-negative status in patients with MRD-positive response. On the contrary, achievement MRD-negative status promoted to increase of PFS. The PFS median in MRD-positive group of patients (n=36: 21 CR, 6 VGPR, 9 PR) was 21 months, in the MRD-negative group (n=16) – 66 months (p=.0068). The PFS median patients with CR was higher in the MRD-negative group than in the MRD-positive group (66 and 48 months, respectively, p=.0045). The tumor load is also a strong prognostic factor like MRD status. Patients who attained low-level MRD had of benefit in the duration of PFS: ˂0.01% - 66 months, 0.01%>0.1% - 48 months at, 0.1%>1% - 22 months, ˃1% - 10 months (p=.0009).

Conclusion
The frequency of achievement MRD-negative doesn’t depend from program of induction therapy, HDT with ASCT and maintenance therapy. Negative prognostic role of MRD status independent from clinical response. Presence of MRD after treatment to associated with decrease of PFS and early relapse. Control of MRD allows to increase of PFS and can be done by means of modern drugs and its combinations, HDT with ASCT and maintenance therapy. Impact of MRD requires further studies, especially after HDT with ASCT.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Survival, Stem cell transplant, Myeloma, Minimal residual disease (MRD)


