PROLONGED THROMBOPROPHYLAXIS IN PATIENTS TREATED WITH LENALIDOMIDE AND DEXAMETHASONE DOES NOT SEEM STRICTLY MANDATORY TO PREVENT LATE THROMBOTIC EVENTS
(Abstract release date: 05/18/17)
EHA Library. Claudio Salvatore C. 05/18/17; 182684; PB1970

Cartia Claudio Salvatore
Contributions
Contributions
Abstract
Abstract: PB1970
Type: Publication Only
Background
Risk of venous thromboembolism (VTE) in general population is 1% annually, significantly higher in oncologic setting, in particular with Multiple Myeloma (MM). Treatment with Lenalidomide plus Dexamethasone represents an additional risk factor for VTE, with most of VTE events observed in the first six months since therapy starting. No definitive data are available on the more appropriate duration of thromboprophylaxis (TP) in patients treated with lenalidomide.
Aims
To explore: I) the incidence of late thrombotic events in a real world population of relapsed MM, addressed to Lenalidomide plus low dose Dexamethasone treatment (Len-dex), and concomitant TP with low molecular weight heparin (LMWH) performed for the first 4-6 months of therapy, without TP maintenance, II) the possible correlation between the presence of thrombotic risk factors and the occurrence of a late VTE.
Methods
We performed a retrospective analysis, after regular approval of local ethic committee, on chart data of 103 patients (pts) with relapsed MM treated with Len-dex according to label indication between January 2003 and December 2016 at our single centre institution. VTE prophylaxis was performed with daily dose of subcutaneous LMWH 4000 IU for 4-6 months, with no further TP, regardless the presence of thrombotic risk factors.
Results
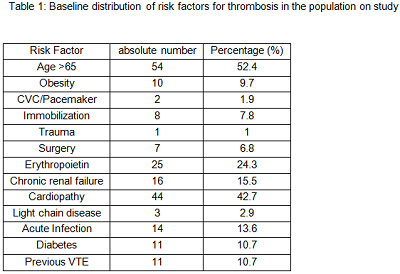
Main features of patients on study were: median age 66.3 years (range 41.9 – 85.2 years), median previous line of therapy 3 (range 1 – 7), time from diagnosis to lenalidomide starting 33.3 months (range 0.3 – 159.9 months), median duration of Lenalidomide treatment 8 months (range 0.4 – 65.2 months) with the following response: ≥PR 56%, CR 7%. Table 1 shows type and distribution of risk factors for VTE. In details median number of VTE risk factors per patient was 2 (range 0-6), 58.2% of pts had ≥2 risk factors, 41.8% of pts (43 pts) had 0-1 risk factor for VTE. Median duration of TP is 4.8 months (range 0.4 – 6 months). No hemorrhagic events were observed during LMWH. Cumulative incidence of VTE was 11.7% (12/103 pts), similar to that previously reported in the literature in patients with continuous TP. The median time from lenalidomide starting and VTE occurrence was of 12.2 months (range 1- 88.2 months), with only one patient developing early VTE among our group. In detail we observed 10 deep vein thrombosis (83%), 1 pulmonary embolism (8.5%), 1 myocardial infarction (8.5%). Most of patients developing VTE had good disease control (≥PR 83%, 10 pts). Concomitant adverse events (AE) was registered in 41.7% of pts (5/12). Most common concomitant AE were infections of respiratory tract (3 pts) and gastrointestinal AE (2 pts). The median number of risk factors for VTE in patients developing or not thrombosis was similar (2.5 vs 2, p=0.092).
Conclusion
This study shows that LMWH is effective and well tolerate for early VTE prophylaxis during Lenalidomide plus low dose Dexamethasone. Incidence of late VTE without TP maintenance is similar to that reported with long-term antiplatelet therapy. We found no difference in factors predisposing for thrombosis among patients developing or not VTE, with a not negligible proportion of concomitant adverse events observed nearby VTE occurrence.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Myeloma, Immunomodulatory thalidomide analog, Thrombosis
Abstract: PB1970
Type: Publication Only
Background
Risk of venous thromboembolism (VTE) in general population is 1% annually, significantly higher in oncologic setting, in particular with Multiple Myeloma (MM). Treatment with Lenalidomide plus Dexamethasone represents an additional risk factor for VTE, with most of VTE events observed in the first six months since therapy starting. No definitive data are available on the more appropriate duration of thromboprophylaxis (TP) in patients treated with lenalidomide.
Aims
To explore: I) the incidence of late thrombotic events in a real world population of relapsed MM, addressed to Lenalidomide plus low dose Dexamethasone treatment (Len-dex), and concomitant TP with low molecular weight heparin (LMWH) performed for the first 4-6 months of therapy, without TP maintenance, II) the possible correlation between the presence of thrombotic risk factors and the occurrence of a late VTE.
Methods
We performed a retrospective analysis, after regular approval of local ethic committee, on chart data of 103 patients (pts) with relapsed MM treated with Len-dex according to label indication between January 2003 and December 2016 at our single centre institution. VTE prophylaxis was performed with daily dose of subcutaneous LMWH 4000 IU for 4-6 months, with no further TP, regardless the presence of thrombotic risk factors.
Results
Main features of patients on study were: median age 66.3 years (range 41.9 – 85.2 years), median previous line of therapy 3 (range 1 – 7), time from diagnosis to lenalidomide starting 33.3 months (range 0.3 – 159.9 months), median duration of Lenalidomide treatment 8 months (range 0.4 – 65.2 months) with the following response: ≥PR 56%, CR 7%. Table 1 shows type and distribution of risk factors for VTE. In details median number of VTE risk factors per patient was 2 (range 0-6), 58.2% of pts had ≥2 risk factors, 41.8% of pts (43 pts) had 0-1 risk factor for VTE. Median duration of TP is 4.8 months (range 0.4 – 6 months). No hemorrhagic events were observed during LMWH. Cumulative incidence of VTE was 11.7% (12/103 pts), similar to that previously reported in the literature in patients with continuous TP. The median time from lenalidomide starting and VTE occurrence was of 12.2 months (range 1- 88.2 months), with only one patient developing early VTE among our group. In detail we observed 10 deep vein thrombosis (83%), 1 pulmonary embolism (8.5%), 1 myocardial infarction (8.5%). Most of patients developing VTE had good disease control (≥PR 83%, 10 pts). Concomitant adverse events (AE) was registered in 41.7% of pts (5/12). Most common concomitant AE were infections of respiratory tract (3 pts) and gastrointestinal AE (2 pts). The median number of risk factors for VTE in patients developing or not thrombosis was similar (2.5 vs 2, p=0.092).

Conclusion
This study shows that LMWH is effective and well tolerate for early VTE prophylaxis during Lenalidomide plus low dose Dexamethasone. Incidence of late VTE without TP maintenance is similar to that reported with long-term antiplatelet therapy. We found no difference in factors predisposing for thrombosis among patients developing or not VTE, with a not negligible proportion of concomitant adverse events observed nearby VTE occurrence.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Myeloma, Immunomodulatory thalidomide analog, Thrombosis
{{ help_message }}
{{filter}}


